Cytokines regulate matrix metalloproteinases and migration in cardiac fibroblasts
- PMID: 17706606
- PMCID: PMC2017114
- DOI: 10.1016/j.bbrc.2007.08.003
Cytokines regulate matrix metalloproteinases and migration in cardiac fibroblasts
Abstract
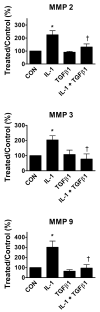
We sought to define the relationship between cytokine stimulated release of matrix metalloproteinases (MMPs) and cell migration using adult rat cardiac fibroblasts. Interleukin-1beta (IL-1beta) increased release of MMP-2, -3, and -9, and TIMP-1, by 3-6-fold, measured by immunoblotting and gel zymography. Tumor necrosis factor-alpha (TNFalpha) augmented IL-1beta stimulated release of MMP-9, but not MMP-2 or -3. Transforming growth factor-beta1 (TGFbeta1) attenuated all the responses to IL-1beta. IL-1beta was also the most robust stimulus of adult rat cardiac fibroblast migration, measured in Boyden chamber assays. The combination of IL-1beta plus TNFalpha substantially enhanced migration, whereas TGFbeta1 strongly inhibited the migratory response to IL-1beta. The pan-selective MMP inhibitor GM 6001 effectively blocked IL-1beta stimulated migration. Pharmacologic inhibitors selective for ERK, JNK, and p38 MAP kinase pathways inhibited the IL-1beta regulation of individual MMPs. Increased MMP activity associated with migration of cardiac fibroblasts may be important determinants of cytokine-directed remodeling of injured myocardium.
Figures



References
-
- Wilson EM, Spinale FG. Myocardial remodelling and matrix metalloproteinases in heart failure: turmoil within the interstitium. Ann Med. 2001;33:623–634. - PubMed
-
- Frangogiannis NG, Smith CW, Entman ML. The inflammatory response in myocardial infarction. Cardiovasc Res. 2002;53:31–47. - PubMed
-
- Leask A, Abraham DJ. TGF-beta signaling and the fibrotic response. FASEB J. 2004;18:816–827. - PubMed
-
- Weber KT, Sun Y, Katwa LC. Wound healing following myocardial infarction. Clin Cardiol. 1996;19:447–455. - PubMed
-
- Eghbali M. Cardiac fibroblasts: function, regulation of gene expression, and phenotypic modulation. Basic Res Cardiol. 1992;87(Suppl 2):183–189. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

