Morphine treatment accelerates sarcoma-induced bone pain, bone loss, and spontaneous fracture in a murine model of bone cancer
- PMID: 17706870
- PMCID: PMC2704581
- DOI: 10.1016/j.pain.2007.06.026
Morphine treatment accelerates sarcoma-induced bone pain, bone loss, and spontaneous fracture in a murine model of bone cancer
Abstract
Metastatic bone cancer causes severe pain that is primarily treated with opioids. A model of bone cancer pain in which the progression of cancer pain and bone destruction is tightly controlled was used to evaluate the effects of sustained morphine treatment. In cancer-treated mice, morphine enhanced, rather than diminished, spontaneous, and evoked pain; these effects were dose-dependent and naloxone-sensitive. SP and CGRP positive DRG cells did not differ between sarcoma or control mice, but were increased following morphine in both groups. Morphine increased ATF-3 expression only in DRG cells of sarcoma mice. Morphine did not alter tumor growth in vitro or tumor burden in vivo but accelerated sarcoma-induced bone destruction and doubled the incidence of spontaneous fracture in a dose- and naloxone-sensitive manner. Morphine increased osteoclast activity and upregulated IL-1 beta within the femurs of sarcoma-treated mice suggesting enhancement of sarcoma-induced osteolysis. These results indicate that sustained morphine increases pain, osteolysis, bone loss, and spontaneous fracture, as well as markers of neuronal damage in DRG cells and expression of pro-inflammatory cytokines. Morphine treatment may result in "add-on" mechanisms of pain beyond those engaged by sarcoma alone. While it is not known whether the present findings in this model of osteolytic sarcoma will generalize to other cancers or opioids, the data suggest a need for increased understanding of neurobiological consequences of prolonged opioid exposure which may allow improvements in the use of opiates in the effective management of cancer pain.
Figures



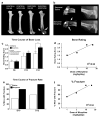
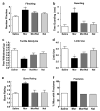

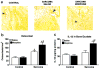
Comment in
-
Of mice and men: what can we predict from the effects of morphine in a mouse model of bone cancer?Pain. 2007 Nov;132(1-2):5-7. doi: 10.1016/j.pain.2007.08.023. Epub 2007 Sep 12. Pain. 2007. PMID: 17854996 No abstract available.
References
-
- Baamonde A, Lastra A, Juarez L, Garcia V, Hidalgo A, Menendez L. Effects of the local administration of selective mu-, delta- and kappa-opioid receptor agonists on osteosarcoma-induced hyperalgesia. Naunyn Schmiedebergs Arch Pharmacol. 2005;372:213–9. - PubMed
-
- Beeton CA, Bord S, Ireland D, Compston JE. Osteoclast formation and bone resorption are inhibited by megakaryocytes. Bone. 2006;39:985–90. - PubMed
-
- Blair HC, Robinson LJ, Zaidi M. Osteoclast signalling pathways. Biochem Biophys Res Commun. 2005;328:728–38. - PubMed
-
- Blum RH, Novetsky D, Shasha D, Fleishman S. The multidisciplinary approach to bone metastases. Oncology (Huntington) 2003;17:845–57. discussion 862–3, p. 867. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

