The Parkinson's disease-associated protein, leucine-rich repeat kinase 2 (LRRK2), is an authentic GTPase that stimulates kinase activity
- PMID: 17706965
- PMCID: PMC2083285
- DOI: 10.1016/j.yexcr.2007.07.007
The Parkinson's disease-associated protein, leucine-rich repeat kinase 2 (LRRK2), is an authentic GTPase that stimulates kinase activity
Abstract
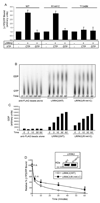
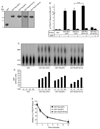
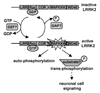
Mutations in the leucine-rich repeat kinase 2 (LRRK2) gene are the leading cause of autosomal dominant Parkinson's disease (PD). LRRK2, a member of the ROCO protein family, contains both Ras GTPase-like (Roc) and kinase (MAPKKK) domains, as well as other functional motifs. Here, we have identified LRRK2 as the first mammalian ROCO protein that is an authentic and functional GTPase, defined by the ability to bind GTP and undergo intrinsic GTP hydrolysis. Furthermore, the Roc domain is sufficient for this native GTPase activity and binds and hydrolyzes GTP indistinguishably from the Ras-related small GTPase, Rac1. The PD-associated mutation, R1441C, located within the Roc domain, leads to an increase in LRRK2 kinase activity and a decrease in the rate of GTP hydrolysis, compared to the wild-type protein, in an in vitro assay. This finding suggests that the R1441C mutation may help stabilize an activated state of LRRK2. Additionally, LRRK2-mediated phosphorylation is stimulated upon binding of non-hydrolyzable GTP analogs, suggesting that LRRK2 is an MAPKKK-activated intramolecularly by its own GTPase. Since GTPases and MAPKKKs are upstream regulators of multiple signal transduction cascades, LRRK2 may play a central role in integrating pathways involved in neuronal cell signaling and the pathogenesis of PD.
Figures


References
-
- Tanner CM, B-S Y. Epidemiology of Parkinson's disease. Adv Neurol. 1999;80:153–159. - PubMed
-
- Moore DJ, W A, Dawson VL, Dawson TM. Molecular pathophysiology of Parkinson's disease. Annu Rev Neurosci. 2005;28:57–87. - PubMed
-
- Cookson MR. The biochemistry of Parkinson's disease. Annu Rev Biochem. 2005;74:29–52. - PubMed
-
- Paisan-Ruiz C, J S, Evans EW, Gilks WP, Simon J, van der Brug M, Lopez de Munain A, Aparicio S, Gil AM, Khan N, Johnson J, Martinez JR, Nicholl D, Carrera IM, Pena AS, de Silva R, Lees A, Marti-Masso JF, Perez-Tur J, Wood NW, Singleton AB. Cloning of the gene containing mutations that cause PARK8-linked Parkinson's disease. Neuron. 2004;44:595–600. - PubMed
-
- Zimprich A, B S, Leitner P, Lichtner P, Farrer M, Lincoln S, Kachergus J, Hulihan M, Uitti RJ, Calne DB, Stoessl AJ, Pfeiffer RF, Patenge N, Carbajal IC, Vieregge P, Asmus F, Muller-Myhsok B, Dickson DW, Meitinger T, Strom TM, Wszolek ZK, Gasser T. Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron. 2004;44:601–607. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

