Initial evaluation of new 99mTc(CO)3 renal imaging agents having carboxyl-rich thioether ligands and chemical characterization of ReCO3 analogues
- PMID: 17707812
- PMCID: PMC2083565
- DOI: 10.1016/j.nucmedbio.2007.06.007
Initial evaluation of new 99mTc(CO)3 renal imaging agents having carboxyl-rich thioether ligands and chemical characterization of ReCO3 analogues
Abstract
Introduction: The first human studies of a characterized radiopharmaceutical containing a {(99m)Tc(CO)(3)}(+) core, Na[(99m)Tc(CO)(3)(LAN)], demonstrated that Na[(99m)Tc(CO)(3)(LAN)] was an excellent renal imaging agent; however, its clearance was less than that of (131)I-orthoiodohippurate ((131)I-OIH), and it did not provide a direct measure of effective renal plasma flow. In order to develop a (99m)Tc renal agent with pharmacokinetic properties equivalent to those of (131)I-OIH, we investigated the (99m)Tc(CO)(3)/Re(CO)(3) complexes formed from carboxymethylmercaptosuccinic acid (CMSAH(3)) and thiodisuccinic acid (TDSAH(4)). Once the ligand is bound to (99m)Tc(CO)(3) through a thioether and two carboxyl groups, the complexes have at least one unbound carboxyl group, essential for the interaction with the renal tubular transporter.
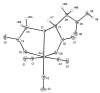
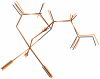
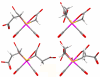
Methods: X-ray crystal structural analysis of [NMe(4)][Re(CO)(3)(CMSAH)] was performed to interpret the nature of (99m)Tc tracers. CMSAH(3) and TDSAH(4) were radiolabeled by incubating each ligand and the precursor [(99m)Tc(CO)(3)(H(2)O)(3)](+) at 70 degrees C (pH 7) for 30 min. The products were purified by reversed-phase high-performance liquid chromatography, and biodistribution studies were performed in Sprague-Dawley rats, with (131)I-OIH as an internal control at 10 and 60 min.
Results: Radiolabeling CMSAH(3) and TDSAH(4) with the [(99m)Tc(CO)(3)(H(2)O)(3)](+) precursor gave products quantitatively. Analysis of the Re(CO)(3) complexes with the CMSAH(3) and TDSAH(4) ligands demonstrates that ligands are bound in (99m)Tc/Re(CO)(3) complexes through a thioether and two deprotonated carboxyl groups (forming tridentate dianionic moieties, generally with two 5-membered chelate rings). Renal excretion at 60 min (activity in the urine as a percentage of (131)I-OIH) was 68+/-1% for Na(3)[(99m)Tc(CO)(3)(TDSA)] but was 98+/-1% for Na(2)[(99m)Tc(CO)(3)(CMSA)].
Conclusion: In rats, Na(2)[(99m)Tc(CO)(3)(CMSA)] is extracted by the kidneys and eliminated in the urine almost as rapidly as (131)I-OIH; consequently, Na(2)[(99m)Tc(CO)(3)(CMSA)] may provide a direct measure of effective renal plasma flow, and further evaluation in humans is warranted.
Figures


Similar articles
-
99mTc(CO)3-nitrilotriacetic acid: a new renal radiopharmaceutical showing pharmacokinetic properties in rats comparable to those of 131I-OIH.J Nucl Med. 2009 Mar;50(3):454-60. doi: 10.2967/jnumed.108.058768. Epub 2009 Feb 17. J Nucl Med. 2009. PMID: 19223406 Free PMC article.
-
First evaluation of a 99mTc-tricarbonyl complex, 99mTc(CO)3(LAN), as a new renal radiopharmaceutical in humans.J Nucl Med. 2006 Jun;47(6):1032-40. J Nucl Med. 2006. PMID: 16741314 Free PMC article. Clinical Trial.
-
Preclinical evaluation of 99mTc(CO)3-aspartic-N-monoacetic acid, a renal radiotracer with pharmacokinetic properties comparable to 131I-o-iodohippurate.J Nucl Med. 2012 Aug;53(8):1277-83. doi: 10.2967/jnumed.111.102236. Epub 2012 Jun 20. J Nucl Med. 2012. PMID: 22717977 Free PMC article.
-
Technetium-99m (99mTc) mercaptoacetyltriglycine: update on the new 99mTc renal tubular function agent.Semin Nucl Med. 1992 Apr;22(2):61-73. doi: 10.1016/s0001-2998(05)80082-0. Semin Nucl Med. 1992. PMID: 1534184 Review.
-
Technetium-99m-EC and other potential new agents in renal nuclear medicine.Semin Nucl Med. 1999 Apr;29(2):91-101. doi: 10.1016/s0001-2998(99)80002-6. Semin Nucl Med. 1999. PMID: 10321823 Review.
Cited by
-
New monodentate amidine superbasic ligands with a single configuration in fac-[Re(CO)3(5,5'- or 6,6'-Me2bipyridine)(amidine)]BF4 complexes.Inorg Chem. 2012 Jul 2;51(13):7271-83. doi: 10.1021/ic300625n. Epub 2012 Jun 12. Inorg Chem. 2012. PMID: 22691073 Free PMC article.
-
99mTc(CO)3-nitrilotriacetic acid: a new renal radiopharmaceutical showing pharmacokinetic properties in rats comparable to those of 131I-OIH.J Nucl Med. 2009 Mar;50(3):454-60. doi: 10.2967/jnumed.108.058768. Epub 2009 Feb 17. J Nucl Med. 2009. PMID: 19223406 Free PMC article.
-
Optimal His-Tag Design for Efficient [99mTc(CO)3]+ and [188Re(CO)3]+ Labeling of Proteins for Molecular Imaging and Radionuclide Therapy by Analysis of Peptide Arrays.Bioconjug Chem. 2021 Jul 21;32(7):1242-1254. doi: 10.1021/acs.bioconjchem.0c00561. Epub 2020 Nov 26. Bioconjug Chem. 2021. PMID: 33241692 Free PMC article.
-
Synthesis and characterization of fac-Re(CO)3-aspartic-N-monoacetic acid, a structural analogue of a potential new renal tracer, fac-99mTc(CO)3(ASMA).Eur J Inorg Chem. 2012 Sep;2012(27):10.1002/ejic.201200599. doi: 10.1002/ejic.201200599. Eur J Inorg Chem. 2012. PMID: 24273448 Free PMC article.
-
Several novel N-donor tridentate ligands formed in chemical studies of new fac-Re(CO)3 complexes relevant to fac-99mTc(CO)3 radiopharmaceuticals: attack of a terminal amine on coordinated acetonitrile.Inorg Chem. 2010 Mar 1;49(5):2123-31. doi: 10.1021/ic901705x. Inorg Chem. 2010. PMID: 20104873 Free PMC article.
References
-
- Vanbilloen HP, Dezutter NA, Cleynhens BJ, Verbruggen AM. Characteristics and biological behaviour of 99mTc-labelled hydroxyacetyltriglycine, a potential alternative to 99mTc-MAG3. Eur J Nucl Med. 1997;24(11):1374–9. - PubMed
-
- Taylor AT, Lipowska M, Hansen L, Malveaux E, Marzilli LG. 99mTc-MAEC complexes: new renal radiopharmaceuticals combining characteristics of 99mTc-MAG3 and 99mTc-EC. J Nucl Med. 2004;45(5):885–91. - PubMed
-
- Jafri RA, Britton KE, Nimmon CC, Solanki K, Alnahhas A, Bomanji J, et al. Technetium-99m-MAG3, a comparison with iodine-123 and iodine-131-orthoiodohippurate, in patients with renal disorders. J Nucl Med. 1988;29(2):147–58. - PubMed
-
- Russell CD, Thorstad B, Yester MV, Stutzman M, Baker T, Dubovsky EV. Comparison of technetium-99m MAG3 with iodine-131-hippuran by a simultaneous dual channel technique. J Nucl Med. 1988;29(7):1189–93. - PubMed
-
- Taylor AT, Ziffer JA, Steves A, Eshima D, Delaney VB, Welchel JD. Clinical comparison of I-131-orthoiodohippurate and the kit formulation of Tc-99mmercaptoacetyltriglycine. Radiology. 1989;170(3):721–5. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

