The IGF axis in baboon pregnancy: placental and systemic responses to feeding 70% global ad libitum diet
- PMID: 17707905
- PMCID: PMC2094102
- DOI: 10.1016/j.placenta.2007.06.011
The IGF axis in baboon pregnancy: placental and systemic responses to feeding 70% global ad libitum diet
Abstract
Information on the influence of poor maternal nutrition on the regulation of responses to pregnancy, placental and fetal growth and development is critical to a better understanding of pregnancy physiology and pathophysiology. We determined normal changes and effects of controlled and monitored moderate nutrient restriction (NR) (global nutrient intake reduced to 70% of food consumed by mothers feeding ad libitum from 0.16 to 0.5 of gestation) in the baboon, on important hematological, biochemical, and hormonal indices of fetal growth and placental function. Serum IGF-I:IGFBP-3 ratio was lower in pregnant than control non-pregnant baboons feeding ad libitum. Serum concentrations of total and free IGF-I were decreased in NR mothers compared with controls (p<0.05). The decrease in fetal IGF-I did not reach significance (p=0.057). Serum IGF-I: IGFBP-3 ratio was decreased by NR in both mothers and fetuses. Maternal serum IGF-II was unchanged by NR. Placental IGF-I mRNA and protein abundance were similarly reduced whereas IGF-II mRNA increased in placental tissue of NR compared to control mothers. Systemic (maternal) and local (placental) IGFBP-1 and IGFBP-3 mRNA and protein abundance were unchanged by NR. Type 1 IGF receptor protein in the syncytiotrophoblast increased in NR. Type 2 IGF receptor protein was present in the stem villi core, and decreased after NR. We conclude that moderate NR in this important non-human primate model significantly disrupts the maternal and placental IGF-IGFBP axis and influences placental expression of this key system at the gene and protein level. Changes observed appear to be directed toward preserving placental growth.
Figures
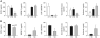



References
-
- Bajoria R, Sooranna SR, Ward S, Hancock M. Placenta as a link between amino acids, insulin-IGF axis, and low birth weight: evidence from twin studies. J Clin Endocrinol Metab. 2002;87:308–15. - PubMed
-
- Harding JE, Liu L, Evans PC, Gluckman PD. Insulin-like growth factor 1 alters feto-placental protein and carbohydrate metabolism in fetal sheep. Endocrinology. 1994;134:1509–14. - PubMed
-
- Miller AG, Aplin JD, Westwood M. Adenovirally mediated expression of insulin-like growth factors enhances the function of first trimester placental fibroblasts. J Clin Endocrinol Metab. 2005;90:379–85. - PubMed
-
- Jansson T, Powell TL. Award in Placentology Lecture. Human placental transport in altered fetal growth: does the placenta function as a nutrient sensor? Placenta. 2006;27(Suppl A):S91–7. Epub 2006 Jan 25 IFPA 2005. - PubMed
-
- Han VK, Carter AM. Spatial and temporal patterns of expression of messenger RNA for insulin-like growth factors and their binding proteins in the placenta of man and laboratory animals. Placenta. 2000;21:289–305. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

