Dissociation of AMP-activated protein kinase and p38 mitogen-activated protein kinase signaling in skeletal muscle
- PMID: 17709097
- PMCID: PMC2040310
- DOI: 10.1016/j.bbrc.2007.07.154
Dissociation of AMP-activated protein kinase and p38 mitogen-activated protein kinase signaling in skeletal muscle
Abstract
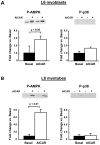
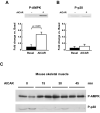
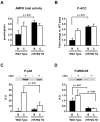
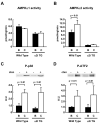
AMP-activated protein kinase (AMPK) is widely recognized as an important regulator of glucose transport in skeletal muscle. The p38 mitogen-activated protein kinase (MAPK) has been proposed to be a component of AMPK-mediated signaling. Here we used several different models of altered AMPK activity to determine whether p38 MAPK is a downstream intermediate of AMPK-mediated signaling in skeletal muscle. First, L6 myoblasts and myotubes were treated with AICAR, an AMPK stimulator. AMPK phosphorylation was significantly increased, but there was no change in p38 MAPK phosphorylation. Similarly, AICAR incubation of isolated rat extensor digitorum longus (EDL) muscles did not increase p38 phosphorylation. Next, we used transgenic mice expressing an inactive form of the AMPKalpha2 catalytic subunit in skeletal muscle (AMPKalpha2i TG mice). AMPKalpha2i TG mice did not exhibit any defect in basal or contraction-induced p38 MAPK phosphorylation. We also used transgenic mice expressing an activating mutation in the AMPKgamma1 subunit (gamma1R70Q TG mice). Despite activated AMPK, basal p38 MAPK phosphorylation was not different between wild type and gamma1R70Q TG mice. In addition, muscle contraction-induced p38 MAPK phosphorylation was significantly blunted in the gamma1R70Q TG mice. In conclusion, increasing AMPK activity by AICAR and AMPKgamma1 mutation does not increase p38 MAPK phosphorylation in skeletal muscle. Furthermore, AMPKalpha2i TG mice lacking contraction-stimulated AMPK activity have normal p38 MAPK phosphorylation. These results suggest that p38 MAPK is not a downstream component of AMPK-mediated signaling in skeletal muscle.
Figures
Similar articles
-
AMP-activated protein kinase alpha2 activity is not essential for contraction- and hyperosmolarity-induced glucose transport in skeletal muscle.J Biol Chem. 2005 Nov 25;280(47):39033-41. doi: 10.1074/jbc.M504208200. Epub 2005 Sep 26. J Biol Chem. 2005. PMID: 16186119
-
The AMP-activated protein kinase activator AICAR does not induce GLUT4 translocation to transverse tubules but stimulates glucose uptake and p38 mitogen-activated protein kinases alpha and beta in skeletal muscle.FASEB J. 2003 Sep;17(12):1658-65. doi: 10.1096/fj.02-1125com. FASEB J. 2003. PMID: 12958172
-
AMP-activated protein kinase activates p38 mitogen-activated protein kinase by increasing recruitment of p38 MAPK to TAB1 in the ischemic heart.Circ Res. 2005 Oct 28;97(9):872-9. doi: 10.1161/01.RES.0000187458.77026.10. Epub 2005 Sep 22. Circ Res. 2005. PMID: 16179588
-
LKB1 and AMPK and the regulation of skeletal muscle metabolism.Curr Opin Clin Nutr Metab Care. 2008 May;11(3):227-32. doi: 10.1097/MCO.0b013e3282fb7b76. Curr Opin Clin Nutr Metab Care. 2008. PMID: 18403917 Free PMC article. Review.
-
AMPK: a key sensor of fuel and energy status in skeletal muscle.Physiology (Bethesda). 2006 Feb;21:48-60. doi: 10.1152/physiol.00044.2005. Physiology (Bethesda). 2006. PMID: 16443822 Review.
Cited by
-
A Potent and Selective AMPK Activator That Inhibits de Novo Lipogenesis.ACS Med Chem Lett. 2010 Aug 30;1(9):478-82. doi: 10.1021/ml100143q. eCollection 2010 Dec 9. ACS Med Chem Lett. 2010. PMID: 24900234 Free PMC article.
-
Localization and regulation of the N terminal splice variant of PGC-1α in adult skeletal muscle fibers.J Biomed Biotechnol. 2012;2012:989263. doi: 10.1155/2012/989263. Epub 2012 Jan 29. J Biomed Biotechnol. 2012. PMID: 22500113 Free PMC article.
-
Stretch-stimulated glucose uptake in skeletal muscle is mediated by reactive oxygen species and p38 MAP-kinase.J Physiol. 2009 Jul 1;587(Pt 13):3363-73. doi: 10.1113/jphysiol.2008.165639. Epub 2009 Apr 29. J Physiol. 2009. PMID: 19403598 Free PMC article.
-
Activation of p38 in C2C12 myotubes following ATP depletion depends on extracellular glucose.J Physiol Biochem. 2015 Jun;71(2):253-65. doi: 10.1007/s13105-015-0406-z. Epub 2015 Apr 4. J Physiol Biochem. 2015. PMID: 25835326
-
Deficiency in TLR4 signal transduction ameliorates cardiac injury and cardiomyocyte contractile dysfunction during ischemia.J Cell Mol Med. 2009 Aug;13(8A):1513-25. doi: 10.1111/j.1582-4934.2009.00798.x. Epub 2009 Jun 5. J Cell Mol Med. 2009. PMID: 19508385 Free PMC article.
References
-
- Hardie DG, Carling D, Carlson M. Annu.Rev.Biochem. 1998;67:821–855. - PubMed
-
- Kemp BE, Mitchelhill KI, Stapleton D, Michell BJ, Chen ZP, Witters LA. Trends Biochem.Sci. 1999;24:22–25. - PubMed
-
- Carling D. Trends Biochem.Sci. 2004;29:18–24. - PubMed
-
- Kahn BB, Alquier T, Carling D, Hardie DG. Cell Metab. 2005;1:15–25. - PubMed
-
- Fujii N, Jessen N, Goodyear LJ. Am J Physiol Endocrinol.Metab. 2006;291:E867–E877. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

