Histone deacetylase inhibitors reduce steroidogenesis through SCF-mediated ubiquitination and degradation of steroidogenic factor 1 (NR5A1)
- PMID: 17709382
- PMCID: PMC2168912
- DOI: 10.1128/MCB.00476-07
Histone deacetylase inhibitors reduce steroidogenesis through SCF-mediated ubiquitination and degradation of steroidogenic factor 1 (NR5A1)
Abstract
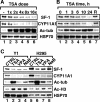
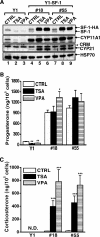
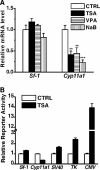
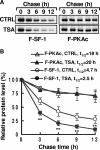
Histone deacetylase (HDAC) inhibitors such as trichostatin A and valproic acid modulate transcription of many genes by inhibiting the activities of HDACs, resulting in the remodeling of chromatin. Yet this effect is not universal for all genes. Here we show that HDAC inhibitors suppressed the expression of steroidogenic gene CYP11A1 and decreased steroid secretion by increasing the ubiquitination and degradation of SF-1, a factor important for the transcription of all steroidogenic genes. This was accompanied by increased expression of Ube2D1 and SKP1A, an E2 ubiquitin conjugase and a subunit of the E3 ubiquitin ligase in the Skp1/Cul1/F-box protein (SCF) family, respectively. Reducing SKP1A expression with small interfering RNA resulted in recovery of SF-1 levels, demonstrating that the activity of SCF E3 ubiquitin ligase is required for the SF-1 degradation induced by HDAC inhibitors. Overexpression of exogenous SF-1 restored steroidogenic activities even in the presence of HDAC inhibitors. Thus, increased SF-1 degradation is the cause of the reduction in steroidogenesis caused by HDAC inhibitors. The increased SKP1A expression and SCF-mediated protein degradation could be the mechanism underlying the mode of action of HDAC inhibitors.
Figures
Similar articles
-
The histone deacetylase inhibitor valproic acid selectively induces proteasomal degradation of HDAC2.EMBO J. 2003 Jul 1;22(13):3411-20. doi: 10.1093/emboj/cdg315. EMBO J. 2003. PMID: 12840003 Free PMC article.
-
SCFFBXO28-mediated self-ubiquitination of FBXO28 promotes its degradation.Cell Signal. 2020 Jan;65:109440. doi: 10.1016/j.cellsig.2019.109440. Epub 2019 Oct 31. Cell Signal. 2020. PMID: 31678254
-
The tumor suppressor ING3 is degraded by SCF(Skp2)-mediated ubiquitin-proteasome system.Oncogene. 2010 Mar 11;29(10):1498-508. doi: 10.1038/onc.2009.424. Epub 2009 Nov 23. Oncogene. 2010. PMID: 19935701
-
A sporadic Parkinson's disease model via silencing of the ubiquitin-proteasome/E3 ligase component, SKP1A.J Neural Transm (Vienna). 2024 Jun;131(6):675-707. doi: 10.1007/s00702-023-02687-6. Epub 2023 Aug 29. J Neural Transm (Vienna). 2024. PMID: 37644186 Free PMC article. Review.
-
The Ubiquitin-Proteasome Pathway and Epigenetic Modifications in Cancer.Anticancer Agents Med Chem. 2021;21(1):20-32. doi: 10.2174/1871520620666200811114159. Anticancer Agents Med Chem. 2021. PMID: 32781973 Review.
Cited by
-
Hedgehog signaling and steroidogenesis.Annu Rev Physiol. 2015;77:105-29. doi: 10.1146/annurev-physiol-061214-111754. Annu Rev Physiol. 2015. PMID: 25668018 Free PMC article. Review.
-
Development of Human Adrenocortical Adenoma (HAA1) Cell Line from Zona Reticularis.Int J Mol Sci. 2022 Dec 29;24(1):584. doi: 10.3390/ijms24010584. Int J Mol Sci. 2022. PMID: 36614027 Free PMC article.
-
Sex-specific Behavioral Features of Rodent Models of Autism Spectrum Disorder.Exp Neurobiol. 2018 Oct;27(5):321-343. doi: 10.5607/en.2018.27.5.321. Epub 2018 Oct 31. Exp Neurobiol. 2018. PMID: 30429643 Free PMC article. Review.
-
GnRH pulse frequency differentially regulates steroidogenic factor 1 (SF1), dosage-sensitive sex reversal-AHC critical region on the X chromosome gene 1 (DAX1), and serum response factor (SRF): potential mechanism for GnRH pulse frequency regulation of LH beta transcription in the rat.Endocrine. 2011 Jun;39(3):212-9. doi: 10.1007/s12020-011-9440-y. Epub 2011 Mar 16. Endocrine. 2011. PMID: 21409515
-
Histone deacetylase inhibitor SAHA epigenetically regulates miR-17-92 cluster and MCM7 to upregulate MICA expression in hepatoma.Br J Cancer. 2015 Jan 6;112(1):112-21. doi: 10.1038/bjc.2014.547. Epub 2014 Nov 13. Br J Cancer. 2015. PMID: 25393367 Free PMC article.
References
-
- Achermann, J. C., M. Ito, M. Ito, P. C. Hindmarsh, and J. L. Jameson. 1999. A mutation in the gene encoding steroidogenic factor-1 causes XY sex reversal and adrenal failure in humans. Nat. Genet. 22:125-126. - PubMed
-
- Alao, J. P., E. W. Lam, S. Ali, L. Buluwela, W. Bordogna, P. Lockey, R. Varshochi, A. V. Stavropoulou, R. C. Coombes, and D. M. Vigushin. 2004. Histone deacetylase inhibitor trichostatin A represses estrogen receptor alpha-dependent transcription and promotes proteasomal degradation of cyclin D1 in human breast carcinoma cell lines. Clin. Cancer Res. 10:8094-8104. - PubMed
-
- Ang, X. L., and J. W. Harper. 2005. SCF-mediated protein degradation and cell cycle control. Oncogene 24:2860-2870. - PubMed
-
- Bland, M. L., R. C. Fowkes, and H. A. Ingraham. 2004. Differential requirement for steroidogenic factor-1 gene dosage in adrenal development versus endocrine function. Mol. Endocrinol. 18:941-952. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
