pRb-dependent cyclin D3 protein stabilization is required for myogenic differentiation
- PMID: 17709384
- PMCID: PMC2168908
- DOI: 10.1128/MCB.02199-06
pRb-dependent cyclin D3 protein stabilization is required for myogenic differentiation
Abstract
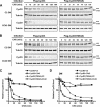
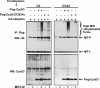
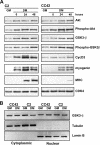
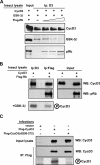
The expression of retinoblastoma (pRb) and cyclin D3 proteins is highly induced during the process of skeletal myoblast differentiation. We have previously shown that cyclin D3 is nearly totally associated with hypophosphorylated pRb in differentiated myotubes, whereas Rb-/- myocytes fail to accumulate the cyclin D3 protein despite normal induction of cyclin D3 mRNA. Here we report that pRb promotes cyclin D3 protein accumulation in differentiating myoblasts by preventing cyclin D3 degradation. We show that cyclin D3 displays rapid turnover in proliferating myoblasts, which is positively regulated through glycogen synthase kinase 3beta (GSK-3beta)-mediated phosphorylation of cyclin D3 on Thr-283. We describe a novel interaction between pRb and cyclin D3 that maps to the C terminus of pRb and to a region of cyclin D3 proximal to the Thr-283 residue and provide evidence that the pRb-cyclin D3 complex formation in terminally differentiated myotubes hinders the access of GSK-3beta to cyclin D3, thus inhibiting Thr-283 phosphorylation. Interestingly, we observed that the ectopic expression of a stabilized cyclin D3 mutant in C2 myoblasts enhances muscle-specific gene expression; conversely, cyclin D3-null embryonic fibroblasts display impaired MyoD-induced myogenic differentiation. These results indicate that the pRb-dependent accumulation of cyclin D3 is functionally relevant to the process of skeletal muscle cell differentiation.
Figures







Similar articles
-
Critical role played by cyclin D3 in the MyoD-mediated arrest of cell cycle during myoblast differentiation.Mol Cell Biol. 1999 Jul;19(7):5203-17. doi: 10.1128/MCB.19.7.5203. Mol Cell Biol. 1999. PMID: 10373569 Free PMC article.
-
Sequestration of pRb by cyclin D3 causes intranuclear reorganization of lamin A/C during muscle cell differentiation.Mol Biol Cell. 2005 Apr;16(4):1948-60. doi: 10.1091/mbc.e04-02-0154. Epub 2005 Feb 9. Mol Biol Cell. 2005. PMID: 15703219 Free PMC article.
-
Ectopic expression of cyclin D3 corrects differentiation of DM1 myoblasts through activation of RNA CUG-binding protein, CUGBP1.Exp Cell Res. 2008 Jul 1;314(11-12):2266-78. doi: 10.1016/j.yexcr.2008.04.018. Epub 2008 May 10. Exp Cell Res. 2008. PMID: 18570922 Free PMC article.
-
Expression and activity of the retinoblastoma protein (pRB)-family proteins, p107 and p130, during L6 myoblast differentiation.Cell Growth Differ. 1995 Oct;6(10):1287-98. Cell Growth Differ. 1995. PMID: 8845306
-
[Interactions of proliferation and differentiation signaling pathways in myogenesis].Postepy Hig Med Dosw (Online). 2014 May 8;68:516-26. doi: 10.5604/17322693.1101617. Postepy Hig Med Dosw (Online). 2014. PMID: 24864103 Review. Polish.
Cited by
-
Retinoblastoma-independent antiproliferative activity of novel intracellular antibodies against the E7 oncoprotein in HPV 16-positive cells.BMC Cancer. 2011 Jan 17;11:17. doi: 10.1186/1471-2407-11-17. BMC Cancer. 2011. PMID: 21241471 Free PMC article.
-
AMP-activated protein kinase regulates beta-catenin transcription via histone deacetylase 5.J Biol Chem. 2011 May 6;286(18):16426-34. doi: 10.1074/jbc.M110.199372. Epub 2011 Mar 17. J Biol Chem. 2011. PMID: 21454484 Free PMC article.
-
GSK3β mediates muscle pathology in myotonic dystrophy.J Clin Invest. 2012 Dec;122(12):4461-72. doi: 10.1172/JCI64081. Epub 2012 Nov 19. J Clin Invest. 2012. PMID: 23160194 Free PMC article.
-
Small nucleolar RNAs promote the restoration of muscle differentiation defects in cells from myotonic dystrophy type 1.Nucleic Acids Res. 2025 Mar 20;53(6):gkaf232. doi: 10.1093/nar/gkaf232. Nucleic Acids Res. 2025. PMID: 40156865 Free PMC article.
-
Non-canonical functions of cell cycle cyclins and cyclin-dependent kinases.Nat Rev Mol Cell Biol. 2016 May;17(5):280-92. doi: 10.1038/nrm.2016.27. Epub 2016 Apr 1. Nat Rev Mol Cell Biol. 2016. PMID: 27033256 Free PMC article. Review.
References
-
- Bartkova, J., J. Lukas, M. Strauss, and J. Bartek. 1998. Cyclin D3: requirement for G1/S transition and high abundance in quiescent tissues suggest a dual role in proliferation and differentiation. Oncogene 17:1027-1037. - PubMed
-
- Berkes, C. A., and S. J. Tapscott. 2005. MyoD and the transcriptional control of myogenesis. Semin. Cell Dev. Biol. 16:585-595. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
