Cross-competition of CD8+ T cells shapes the immunodominance hierarchy during boost vaccination
- PMID: 17709425
- PMCID: PMC2118691
- DOI: 10.1084/jem.20070489
Cross-competition of CD8+ T cells shapes the immunodominance hierarchy during boost vaccination
Abstract
CD8+ T cell responses directed against multiple pathogen-derived epitopes are characterized by defined immunodominance hierarchy patterns. A possible explanation for this phenomenon is that CD8+ T cells of different specificities compete for access to epitopes on antigen-presenting cells, and that the outcome of this so-called cross-competition reflects the number of induced T cells. In our study using a vaccinia virus infection model, we found that T cell cross-competition is highly relevant during boost vaccination, thereby shaping the immunodominance hierarchy in the recall. We demonstrate that competition was of no importance during priming and was unaffected by the applied route of immunization. It strongly depended on the timing of viral antigen expression in infected APCs, and it was characterized by poor proliferation of T cells recognizing epitopes derived from late viral proteins. To our knowledge, this is the first demonstration of the functional importance of T cell cross-competition during a viral infection. Our findings provide a basis for novel strategies for how boost vaccination to defined antigens can be selectively improved. They give important new insights into the design of more efficient poxviral vectors for immunotherapy.
Figures

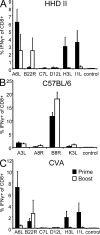



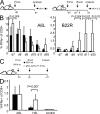
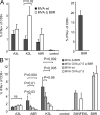

References
-
- Yewdell, J.W., and J.R. Bennink. 1999. Immunodominance in major histocompatibility complex class I-restricted T lymphocyte responses. Annu. Rev. Immunol. 17:51–88. - PubMed
-
- Kedl, R.M., J.W. Kappler, and P. Marrack. 2003. Epitope dominance, competition and T cell affinity maturation. Curr. Opin. Immunol. 15:120–127. - PubMed
-
- Stock, A.T., C.M. Jones, W.R. Heath, and F.R. Carbone. 2006. CTL response compensation for the loss of an immunodominant class I-restricted HSV-1 determinant. Immunol. Cell Biol. 84:543–550. - PubMed
-
- Thomas, P.G., S.A. Brown, R. Keating, W. Yue, M.Y. Morris, J. So, R.J. Webby, and P.C. Doherty. 2007. Hidden epitopes emerge in secondary influenza virus-specific CD8+ T cell responses. J. Immunol. 178:3091–3098. - PubMed
-
- Probst, H.C., T. Dumrese, and M.F. van den Broek. 2002. Cutting edge: competition for APC by CTLs of different specificities is not functionally important during induction of antiviral responses. J. Immunol. 168:5387–5391. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

