Acute loss of intestinal CD4+ T cells is not predictive of simian immunodeficiency virus virulence
- PMID: 17709518
- PMCID: PMC2367134
- DOI: 10.4049/jimmunol.179.5.3035
Acute loss of intestinal CD4+ T cells is not predictive of simian immunodeficiency virus virulence
Abstract
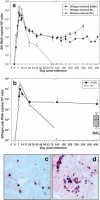
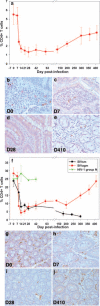
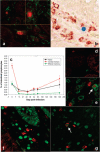
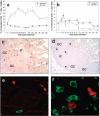
The predictive value of acute gut-associated lymphoid tissue (GALT) CD4+ T cell depletion in lentiviral infections was assessed by comparing three animal models illustrative of the outcomes of SIV infection: pathogenic infection (SIVsmm infection of rhesus macaques (Rh)), persistent nonprogressive infection (SIVagm infection of African green monkeys (AGM)), and transient, controlled infection (SIVagm infection of Rh). Massive acute depletion of GALT CD4+ T cells was a common feature of acute SIV infection in all three models. The outcome of this mucosal CD4+ T cell depletion, however, differed substantially between the three models: in SIVsmm-infected Rh, the acute GALT CD4+ T cell depletion was persistent and continued with disease progression; in SIVagm, intestinal CD4+ T cells were partially restored during chronic infection in the context of normal levels of apoptosis and immune activation and absence of damage to the mucosal immunologic barrier; in SIVagm-infected Rh, complete control of viral replication resulted in restoration of the mucosal barrier and immune restoration. Therefore, our data support a revised paradigm wherein severe GALT CD4+ T cell depletion during acute pathogenic HIV and SIV infections of humans and Rh is necessary but neither sufficient nor predictive of disease progression, with levels of immune activation, proliferation and apoptosis being key factors involved in determining progression to AIDS.
Figures



Similar articles
-
The Hitchhiker Guide to CD4+ T-Cell Depletion in Lentiviral Infection. A Critical Review of the Dynamics of the CD4+ T Cells in SIV and HIV Infection.Front Immunol. 2021 Jul 21;12:695674. doi: 10.3389/fimmu.2021.695674. eCollection 2021. Front Immunol. 2021. PMID: 34367156 Free PMC article.
-
Elite Control, Gut CD4 T Cell Sparing, and Enhanced Mucosal T Cell Responses in Macaca nemestrina Infected by a Simian Immunodeficiency Virus Lacking a gp41 Trafficking Motif.J Virol. 2015 Oct;89(20):10156-75. doi: 10.1128/JVI.01134-15. Epub 2015 Jul 29. J Virol. 2015. PMID: 26223646 Free PMC article.
-
Antiviral therapy during primary simian immunodeficiency virus infection fails to prevent acute loss of CD4+ T cells in gut mucosa but enhances their rapid restoration through central memory T cells.J Virol. 2008 Apr;82(8):4016-27. doi: 10.1128/JVI.02164-07. Epub 2008 Feb 13. J Virol. 2008. PMID: 18272585 Free PMC article.
-
Pathogenic features associated with increased virulence upon Simian immunodeficiency virus cross-species transmission from natural hosts.J Virol. 2014 Jun;88(12):6778-92. doi: 10.1128/JVI.03785-13. Epub 2014 Apr 2. J Virol. 2014. PMID: 24696477 Free PMC article.
-
Walk on the wild side: SIV infection in African non-human primate hosts-from the field to the laboratory.Front Immunol. 2023 Jan 12;13:1060985. doi: 10.3389/fimmu.2022.1060985. eCollection 2022. Front Immunol. 2023. PMID: 36713371 Free PMC article. Review.
Cited by
-
Blocking TLR7- and TLR9-mediated IFN-α production by plasmacytoid dendritic cells does not diminish immune activation in early SIV infection.PLoS Pathog. 2013;9(7):e1003530. doi: 10.1371/journal.ppat.1003530. Epub 2013 Jul 25. PLoS Pathog. 2013. PMID: 23935491 Free PMC article.
-
T cell activation is insufficient to drive SIV disease progression.JCI Insight. 2023 Jul 24;8(14):e161111. doi: 10.1172/jci.insight.161111. JCI Insight. 2023. PMID: 37485874 Free PMC article.
-
Th17 cells and regulatory T cells in elite control over HIV and SIV.Curr Opin HIV AIDS. 2011 May;6(3):221-7. doi: 10.1097/COH.0b013e32834577b3. Curr Opin HIV AIDS. 2011. PMID: 21399494 Free PMC article. Review.
-
Inefficient Nef-mediated downmodulation of CD3 and MHC-I correlates with loss of CD4+T cells in natural SIV infection.PLoS Pathog. 2008 Jul 18;4(7):e1000107. doi: 10.1371/journal.ppat.1000107. PLoS Pathog. 2008. PMID: 18636106 Free PMC article.
-
Rabies virus-based vaccines elicit neutralizing antibodies, poly-functional CD8+ T cell, and protect rhesus macaques from AIDS-like disease after SIV(mac251) challenge.Vaccine. 2009 Dec 11;28(2):299-308. doi: 10.1016/j.vaccine.2009.10.051. Epub 2009 Oct 29. Vaccine. 2009. PMID: 19879223 Free PMC article.
References
-
- Hirsch VM, Johnson PR. Pathogenic diversity of simian immunodeficiency viruses. Virus Res. 1994;32:183–203. - PubMed
-
- Pandrea I, Silvestri G, Onanga R, Veazey RS, Marx PA, Hirsch VM, Apetrei C. Simian immunodeficiency viruses replication dynamics in African non-human primate hosts: common patterns and species-specific differences. J. Med. Primatol. 2006;35:194–201. - PubMed
-
- Pandrea I, Apetrei C, Dufour J, Dillon N, Barbercheck J, Metzger M, Jacquelin B, Bohm R, Marx PA, Barre-Sinoussi F, Hirsch VM, Muller-Trutwin MC, Lackner AA, Veazey R. Simian immunodeficiency virus (SIV) SIVagm.sab infection of Caribbean African green monkeys: new model of the study of SIV pathogenesis in natural hosts. J. Virol. 2006;80:4858–4867. - PMC - PubMed
-
- Pandrea I, Onanga R, Kornfeld C, Rouquet P, Bourry O, Clifford S, Telfer PT, Abernethy K, White LT, Ngari P, et al. High levels of SIVmnd-1 replication in chronically infected Mandrillus sphinx. Virology. 2003;317:119–127. - PubMed
Publication types
MeSH terms
Grants and funding
- P51 RR 000164/RR/NCRR NIH HHS/United States
- R01 AI028433/AI/NIAID NIH HHS/United States
- R01 RR006555/RR/NCRR NIH HHS/United States
- AI 28433/AI/NIAID NIH HHS/United States
- P20 RR 020159/RR/NCRR NIH HHS/United States
- Z99 AI999999/ImNIH/Intramural NIH HHS/United States
- R01 AI 49080/AI/NIAID NIH HHS/United States
- R01 AI 065325/AI/NIAID NIH HHS/United States
- R01 AI 064066/AI/NIAID NIH HHS/United States
- ZIA AI001029/ImNIH/Intramural NIH HHS/United States
- R01 AI064066/AI/NIAID NIH HHS/United States
- R21 AI 069935/AI/NIAID NIH HHS/United States
- P51 RR000164/RR/NCRR NIH HHS/United States
- R37 AI028433/AI/NIAID NIH HHS/United States
- R01 AI065325/AI/NIAID NIH HHS/United States
- R21 AI069935/AI/NIAID NIH HHS/United States
- R01 AI049080/AI/NIAID NIH HHS/United States
- P20 RR020159/RR/NCRR NIH HHS/United States
- RR 18745/RR/NCRR NIH HHS/United States
- RR 06555/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

