Inhibition of HIV-1 infectivity and epithelial cell transfer by human monoclonal IgG and IgA antibodies carrying the b12 V region
- PMID: 17709529
- PMCID: PMC2881690
- DOI: 10.4049/jimmunol.179.5.3144
Inhibition of HIV-1 infectivity and epithelial cell transfer by human monoclonal IgG and IgA antibodies carrying the b12 V region
Abstract
Both IgG and secretory IgA Abs in mucosal secretions have been implicated in blocking the earliest events in HIV-1 transit across epithelial barriers, although the mechanisms by which this occurs remain largely unknown. In this study, we report the production and characterization of a human rIgA(2) mAb that carries the V regions of IgG1 b12, a potent and broadly neutralizing anti-gp120 Ab which has been shown to protect macaques against vaginal simian/HIV challenge. Monomeric, dimeric, polymeric, and secretory IgA(2) derivatives of b12 reacted with gp120 and neutralized CCR5- and CXCR4-tropic strains of HIV-1 in vitro. With respect to the protective effects of these Abs at mucosal surfaces, we demonstrated that IgG1 b12 and IgA(2) b12 inhibited the transfer of cell-free HIV-1 from ME-180 cells, a human cervical epithelial cell line, as well as Caco-2 cells, a human colonic epithelial cell line, to human PBMCs. Inhibition of viral transfer was due to the ability of b12 to block both viral attachment to and uptake by epithelial cells. These data demonstrate that IgG and IgA MAbs directed against a highly conserved epitope on gp120 can interfere with the earliest steps in HIV-1 transmission across mucosal surfaces, and reveal a possible mechanism by which b12 protects the vaginal mucosal against viral challenge in vivo.
Conflict of interest statement
The authors have no financial conflict of interest.
Figures


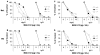
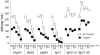
Similar articles
-
Simplifying the synthesis of SIgA: combination of dIgA and rhSC using affinity chromatography.Methods. 2014 Jan 1;65(1):127-32. doi: 10.1016/j.ymeth.2013.06.022. Epub 2013 Jun 25. Methods. 2014. PMID: 23811333 Free PMC article.
-
Identification and characterization of a new cross-reactive human immunodeficiency virus type 1-neutralizing human monoclonal antibody.J Virol. 2004 Sep;78(17):9233-42. doi: 10.1128/JVI.78.17.9233-9242.2004. J Virol. 2004. PMID: 15308718 Free PMC article.
-
Efficient isolation of novel human monoclonal antibodies with neutralizing activity against HIV-1 from transgenic mice expressing human Ig loci.J Immunol. 2002 Jul 1;169(1):595-605. doi: 10.4049/jimmunol.169.1.595. J Immunol. 2002. PMID: 12077293
-
Efficient single tobamoviral vector-based bioproduction of broadly neutralizing anti-HIV-1 monoclonal antibody VRC01 in Nicotiana benthamiana plants and utility of VRC01 in combination microbicides.Antimicrob Agents Chemother. 2013 May;57(5):2076-86. doi: 10.1128/AAC.02588-12. Epub 2013 Feb 12. Antimicrob Agents Chemother. 2013. PMID: 23403432 Free PMC article.
-
Protection of neonatal macaques against experimental SHIV infection by human neutralizing monoclonal antibodies.Transfus Clin Biol. 2001 Aug;8(4):350-8. doi: 10.1016/s1246-7820(01)00187-2. Transfus Clin Biol. 2001. PMID: 11642027 Review.
Cited by
-
Anti-gp120 minibody gene transfer to female genital epithelial cells protects against HIV-1 virus challenge in vitro.PLoS One. 2011;6(10):e26473. doi: 10.1371/journal.pone.0026473. Epub 2011 Oct 21. PLoS One. 2011. PMID: 22031835 Free PMC article.
-
IgA and FcαRI: Pathological Roles and Therapeutic Opportunities.Front Immunol. 2019 Mar 22;10:553. doi: 10.3389/fimmu.2019.00553. eCollection 2019. Front Immunol. 2019. PMID: 30984170 Free PMC article. Review.
-
Isotype modulates epitope specificity, affinity, and antiviral activities of anti-HIV-1 human broadly neutralizing 2F5 antibody.Proc Natl Acad Sci U S A. 2012 Jul 31;109(31):12680-5. doi: 10.1073/pnas.1200024109. Epub 2012 Jun 20. Proc Natl Acad Sci U S A. 2012. PMID: 22723360 Free PMC article.
-
Comparative Evaluation of HIV-1 Neutralization in External Secretions and Sera of HIV-1-Infected Women.Open AIDS J. 2012;6:293-302. doi: 10.2174/1874613601206010293. Epub 2012 Dec 28. Open AIDS J. 2012. PMID: 23346267 Free PMC article.
-
Anti-HIV-1 response elicited in rabbits by anti-idiotype monoclonal antibodies mimicking the CD4-binding site.PLoS One. 2008;3(10):e3423. doi: 10.1371/journal.pone.0003423. Epub 2008 Oct 16. PLoS One. 2008. PMID: 18923648 Free PMC article.
References
-
- Kozlowski PA, Neutra MR. The role of mucosal immunity in prevention of HIV transmission. Curr Mol Med. 2003;3:217–228. - PubMed
-
- Shattock RJ, Moore JP. Inhibiting sexual transmission of HIV-1 infection. Nat Rev Microbiol. 2003;1:25–34. - PubMed
-
- Meng G, Wei X, Wu X, Sellers MT, Decker JM, Moldoveanu Z, Orenstein JM, Graham MF, Kappes JC, Mestecky J, et al. Primary intestinal epithelial cells selectively transfer R5 HIV-1 to CCR5+ cells. Nat Med. 2002;8:150–156. - PubMed
-
- Brenchley JM, Price DA, Douek DC. HIV disease: fallout from a mucosal catastrophe? Nat Immunol. 2006;7:235–239. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HD 41361/HD/NICHD NIH HHS/United States
- AI 33292/AI/NIAID NIH HHS/United States
- R01 AI055332/AI/NIAID NIH HHS/United States
- F32 AI010009/AI/NIAID NIH HHS/United States
- AI 48796/AI/NIAID NIH HHS/United States
- R37 AI033292/AI/NIAID NIH HHS/United States
- R01 AI033292/AI/NIAID NIH HHS/United States
- P30 AI 054907/AI/NIAID NIH HHS/United States
- K01 DK059295/DK/NIDDK NIH HHS/United States
- DK 059295/DK/NIDDK NIH HHS/United States
- AI 55332/AI/NIAID NIH HHS/United States
- R01 HD041361/HD/NICHD NIH HHS/United States
- P30 AI054907/AI/NIAID NIH HHS/United States
- AI 034757/AI/NIAID NIH HHS/United States
- R01 AI048796/AI/NIAID NIH HHS/United States
- R37 AI055332/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

