Characterization of the role of the Synaptotagmin family as calcium sensors in facilitation and asynchronous neurotransmitter release
- PMID: 17709738
- PMCID: PMC1955806
- DOI: 10.1073/pnas.0706711104
Characterization of the role of the Synaptotagmin family as calcium sensors in facilitation and asynchronous neurotransmitter release
Abstract
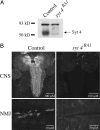
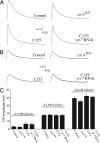
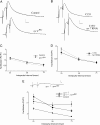
Ca(2+) influx into presynaptic nerve terminals activates synaptic vesicle exocytosis by triggering fast synchronous fusion and a slower asynchronous release pathway. In addition, a brief rise in Ca(2+) after consecutive action potentials has been correlated with a form of short-term synaptic plasticity with enhanced vesicle fusion termed facilitation. Although the synaptic vesicle protein Synaptotagmin 1 (Syt1) has been implicated as the Ca(2+) sensor for synchronous fusion, the molecular identity of the Ca(2+) sensors that mediate facilitation and asynchronous release is unknown. To test whether the synchronous Ca(2+) sensor, Syt1, or the asynchronous Ca(2+) sensor is involved in facilitation, we analyzed whether genetic elimination of Syt1 in Drosophila results in a concomitant impairment in facilitation. Our results indicate that Syt1 acts as a redundant Ca(2+) sensor for facilitation, with the asynchronous Ca(2+) sensor contributing significantly to this form of short-term plasticity. We next examined whether other members of the Drosophila Syt family functioned in Ca(2+)-dependent asynchronous release or facilitation in vivo. Genetic elimination of other panneuronally expressed Syt proteins did not alter these forms of exocytosis, indicating a non-Syt Ca(2+) sensor functions for both facilitation and asynchronous release. In light of these findings, the presence of two presynaptic Ca(2+) sensors can be placed in a biological context, a Syt1-based Ca(2+) sensor devoted primarily to baseline synaptic transmission and a second non-Syt Ca(2+) sensor for short-term synaptic plasticity and asynchronous release.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Differential regulation of synchronous versus asynchronous neurotransmitter release by the C2 domains of synaptotagmin 1.Proc Natl Acad Sci U S A. 2010 Aug 17;107(33):14869-74. doi: 10.1073/pnas.1000606107. Epub 2010 Aug 2. Proc Natl Acad Sci U S A. 2010. PMID: 20679236 Free PMC article.
-
Transmembrane tethering of synaptotagmin to synaptic vesicles controls multiple modes of neurotransmitter release.Proc Natl Acad Sci U S A. 2015 Mar 24;112(12):3793-8. doi: 10.1073/pnas.1420312112. Epub 2015 Mar 9. Proc Natl Acad Sci U S A. 2015. PMID: 25775572 Free PMC article.
-
Drosophila Synaptotagmin 7 negatively regulates synaptic vesicle release and replenishment in a dosage-dependent manner.Elife. 2020 Apr 28;9:e55443. doi: 10.7554/eLife.55443. Elife. 2020. PMID: 32343229 Free PMC article.
-
Function of Drosophila Synaptotagmins in membrane trafficking at synapses.Cell Mol Life Sci. 2021 May;78(9):4335-4364. doi: 10.1007/s00018-021-03788-9. Epub 2021 Feb 22. Cell Mol Life Sci. 2021. PMID: 33619613 Free PMC article. Review.
-
Exocytosis and synaptic vesicle function.Compr Physiol. 2014 Jan;4(1):149-75. doi: 10.1002/cphy.c130021. Compr Physiol. 2014. PMID: 24692137 Review.
Cited by
-
Transmission, Development, and Plasticity of Synapses.Genetics. 2015 Oct;201(2):345-75. doi: 10.1534/genetics.115.176529. Genetics. 2015. PMID: 26447126 Free PMC article. Review.
-
Complexin and Ca2+ stimulate SNARE-mediated membrane fusion.Nat Struct Mol Biol. 2008 Jul;15(7):707-13. doi: 10.1038/nsmb.1446. Epub 2008 Jun 15. Nat Struct Mol Biol. 2008. PMID: 18552825 Free PMC article.
-
Molecular underpinnings of synaptic vesicle pool heterogeneity.Traffic. 2015 Apr;16(4):338-64. doi: 10.1111/tra.12262. Traffic. 2015. PMID: 25620674 Free PMC article. Review.
-
The Mechanisms and Functions of Synaptic Facilitation.Neuron. 2017 May 3;94(3):447-464. doi: 10.1016/j.neuron.2017.02.047. Neuron. 2017. PMID: 28472650 Free PMC article. Review.
-
Doc2b Ca2+ binding site mutants enhance synaptic release at rest at the expense of sustained synaptic strength.Sci Rep. 2019 Oct 8;9(1):14408. doi: 10.1038/s41598-019-50684-1. Sci Rep. 2019. PMID: 31594980 Free PMC article.
References
-
- Katz B. The Release of Neural Transmitter Substances. Liverpool: Liverpool Univ Press; 1969.
-
- Geppert M, Goda Y, Hammer RE, Li C, Rosahl TW, Stevens CF, Sudhof TC. Cell. 1994;79:717–727. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

