Genetic inhibition of cardiac ERK1/2 promotes stress-induced apoptosis and heart failure but has no effect on hypertrophy in vivo
- PMID: 17709754
- PMCID: PMC1955824
- DOI: 10.1073/pnas.0610906104
Genetic inhibition of cardiac ERK1/2 promotes stress-induced apoptosis and heart failure but has no effect on hypertrophy in vivo
Abstract
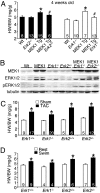
MAPK signaling pathways function as critical regulators of cellular differentiation, proliferation, stress responsiveness, and apoptosis. One branch of the MAPK signaling pathway that culminates in ERK1/2 activation is hypothesized to regulate the growth and adaptation of the heart to both physiologic and pathologic stimuli, given its known activation in response to virtually every stress- and agonist-induced hypertrophic stimulus examined to date. Here we investigated the requirement of ERK1/2 signaling in mediating the cardiac hypertrophic growth response in Erk1(-/-) and Erk2(+/-) mice, as well as in transgenic mice with inducible expression of an ERK1/2-inactivating phosphatase in the heart, dual-specificity phosphatase 6. Although inducible expression of dual-specificity phosphatase 6 in the heart eliminated ERK1/2 phosphorylation at baseline and after stimulation without affecting any other MAPK, it did not diminish the hypertrophic response to pressure overload stimulation, neuroendocrine agonist infusion, or exercise. Similarly, Erk1(-/-) and Erk2(+/-) mice showed no reduction in pathologic or physiologic stimulus-induced cardiac growth in vivo. However, blockade or deletion of cardiac ERK1/2 did predispose the heart to decompensation and failure after long-term pressure overload in conjunction with an increase in myocyte TUNEL. Thus, ERK1/2 signaling is not required for mediating physiologic or pathologic cardiac hypertrophy in vivo, although it does play a protective role in response to pathologic stimuli.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

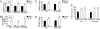


References
-
- Ho KK, Levy D, Kannel WB, Pinsky JL. J Am Coll Cardiol. 1993;22:6–13. - PubMed
-
- Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. N Engl J Med. 1990;322:1561–1566. - PubMed
-
- Dickhuth HH, Lehmann M, Auch-Schwelk W, Meinertz T, Keul J. J Cardiovasc Pharmacol. 1987;10:S71–S78. - PubMed
-
- Allen DL, Harrison BC, Maass A, Bell ML, Byrnes WC, Leinwand LA. J Appl Physiol. 2001;90:1900–1908. - PubMed
-
- Heineke J, Molkentin JD. Nat Rev Mol Cell Biol. 2006;7:589–600. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

