Possible role of spinal astrocytes in maintaining chronic pain sensitization: review of current evidence with focus on bFGF/JNK pathway
- PMID: 17710215
- PMCID: PMC1949390
- DOI: 10.1017/S1740925X07000403
Possible role of spinal astrocytes in maintaining chronic pain sensitization: review of current evidence with focus on bFGF/JNK pathway
Abstract
Although pain is regarded traditionally as neuronally mediated, recent progress shows an important role of spinal glial cells in persistent pain sensitization. Mounting evidence has implicated spinal microglia in the development of chronic pain (e.g. neuropathic pain after peripheral nerve injury). Less is known about the role of astrocytes in pain regulation. However, astrocytes have very close contact with synapses and maintain homeostasis in the extracellular environment. In this review, we provide evidence to support a role of spinal astrocytes in maintaining chronic pain. In particular, c-Jun N-terminal kinase (JNK) is activated persistently in spinal astrocytes in a neuropathic pain condition produced by spinal nerve ligation. This activation is required for the maintenance of neuropathic pain because spinal infusion of JNK inhibitors can reverse mechanical allodynia, a major symptom of neuropathic pain. Further study reveals that JNK is activated strongly in astrocytes by basic fibroblast growth factor (bFGF), an astroglial activator. Intrathecal infusion of bFGF also produces persistent mechanical allodynia. After peripheral nerve injury, bFGF might be produced by primary sensory neurons and spinal astrocytes because nerve injury produces robust bFGF upregulation in both cell types. Therefore, the bFGF/JNK pathway is an important signalling pathway in spinal astrocytes for chronic pain sensitization. Investigation of signaling mechanisms in spinal astrocytes will identify new molecular targets for the management of chronic pain.
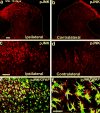
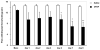
Figures




Similar articles
-
A peptide c-Jun N-terminal kinase (JNK) inhibitor blocks mechanical allodynia after spinal nerve ligation: respective roles of JNK activation in primary sensory neurons and spinal astrocytes for neuropathic pain development and maintenance.J Neurosci. 2006 Mar 29;26(13):3551-60. doi: 10.1523/JNEUROSCI.5290-05.2006. J Neurosci. 2006. PMID: 16571763 Free PMC article.
-
JNK-induced MCP-1 production in spinal cord astrocytes contributes to central sensitization and neuropathic pain.J Neurosci. 2009 Apr 1;29(13):4096-108. doi: 10.1523/JNEUROSCI.3623-08.2009. J Neurosci. 2009. PMID: 19339605 Free PMC article.
-
Elevation of Microglial Basic Fibroblast Growth Factor Contributes to Development of Neuropathic Pain after Spinal Nerve Ligation in Rats.Spine (Phila Pa 1976). 2016 Feb;41(3):E108-15. doi: 10.1097/BRS.0000000000001131. Spine (Phila Pa 1976). 2016. PMID: 26583471
-
Activation of JNK pathway in persistent pain.Neurosci Lett. 2008 Jun 6;437(3):180-3. doi: 10.1016/j.neulet.2008.03.017. Epub 2008 Mar 13. Neurosci Lett. 2008. PMID: 18455869 Free PMC article. Review.
-
Targeting astrocyte signaling for chronic pain.Neurotherapeutics. 2010 Oct;7(4):482-93. doi: 10.1016/j.nurt.2010.05.016. Neurotherapeutics. 2010. PMID: 20880510 Free PMC article. Review.
Cited by
-
Acupuncture Relieves Cervical Spondylosis Radiculopathy by Regulating Spinal Microglia Activation Through MAPK Signaling Pathway in Rats.J Pain Res. 2023 Nov 20;16:3945-3960. doi: 10.2147/JPR.S419927. eCollection 2023. J Pain Res. 2023. PMID: 38026466 Free PMC article.
-
Traumatic brain injury, neuroinflammation, and post-traumatic headaches.Headache. 2013 Oct;53(9):1523-30. doi: 10.1111/head.12173. Epub 2013 Jul 8. Headache. 2013. PMID: 24090534 Free PMC article. Review.
-
Preclinical Assessment of Inflammatory Pain.CNS Neurosci Ther. 2016 Feb;22(2):88-101. doi: 10.1111/cns.12486. Epub 2015 Dec 10. CNS Neurosci Ther. 2016. PMID: 26663896 Free PMC article. Review.
-
K+ Channels in Primary Afferents and Their Role in Nerve Injury-Induced Pain.Front Cell Neurosci. 2020 Sep 17;14:566418. doi: 10.3389/fncel.2020.566418. eCollection 2020. Front Cell Neurosci. 2020. PMID: 33093824 Free PMC article. Review.
-
Light touch induces ERK activation in superficial dorsal horn neurons after inflammation: involvement of spinal astrocytes and JNK signaling in touch-evoked central sensitization and mechanical allodynia.J Neurochem. 2010 Oct;115(2):505-14. doi: 10.1111/j.1471-4159.2010.06946.x. Epub 2010 Aug 31. J Neurochem. 2010. PMID: 20722971 Free PMC article.
References
-
- Aldskogius H, Kozlova EN. Central neuron-glial and glial-glial interactions following axon injury. Progress in Neurobology. 1998;55:1–26. - PubMed
-
- Borsello T, Bonny C. Use of cell-permeable peptides to prevent neuronal degeneration. Trends in Molecular Medicine. 2004;10:239–244. - PubMed
-
- Borsello T, Clarke PG, Hirt L, Vercelli A, Repici M, Schorderet DF, et al. A peptide inhibitor of c-Jun N-terminal kinase protects against excitotoxicity and cerebral ischemia. Molecular Medicine. 2003;9:1180–1186. - PubMed
-
- Colburn RW, DeLeo JA, Rickman AJ, Yeager MP, Kwon P, Hickey WF. Dissociation of microglial activation and neuropathic pain behaviors following peripheral nerve injury in the rat. Journal of Neuroimmunology. 1997;79:163–175. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
