Sequential ABL kinase inhibitor therapy selects for compound drug-resistant BCR-ABL mutations with altered oncogenic potency
- PMID: 17710227
- PMCID: PMC1940237
- DOI: 10.1172/JCI30890
Sequential ABL kinase inhibitor therapy selects for compound drug-resistant BCR-ABL mutations with altered oncogenic potency
Abstract
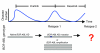
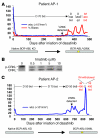
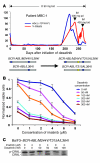
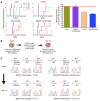
Molecularly targeted kinase inhibitor cancer therapies are currently administered sequentially rather than simultaneously. We addressed the potential long-term impact of this strategy in patients with chronic myelogenous leukemia (CML), which is driven by the fusion oncogene BCR-ABL. Analysis of BCR-ABL genotypes in CML patients who relapsed after sequential treatment with the ABL inhibitors imatinib and dasatinib revealed evolving resistant BCR-ABL kinase domain mutations in all cases. Twelve patients relapsed with the pan-resistant T315I mutation, whereas 6 patients developed novel BCR-ABL mutations predicted to retain sensitivity to imatinib based on in vitro studies. Three of these patients were retreated with imatinib (or the chemically related compound nilotinib) and responded; however, selection for compound mutants (2 or 3 BCR-ABL mutations in the same molecule) can substantially limit the potential effectiveness of retreating patients with inhibitors that have previously failed. Furthermore, drug-resistant mutations, when compounded, can increase oncogenic potency relative to the component mutants in transformation assays. The Aurora kinase inhibitor VX-680, currently under clinical evaluation based on its activity against the T315I mutation, is also effective against the other commonly detected dasatinib-resistant mutation in our analysis, V299L. Our findings demonstrate the potential hazards of sequential kinase inhibitor therapy and suggest a role for a combination of ABL kinase inhibitors, perhaps including VX-680, to prevent the outgrowth of cells harboring drug-resistant BCR-ABL mutations.
Figures

Similar articles
-
The ABL switch control inhibitor DCC-2036 is active against the chronic myeloid leukemia mutant BCR-ABLT315I and exhibits a narrow resistance profile.Cancer Res. 2011 May 1;71(9):3189-95. doi: 10.1158/0008-5472.CAN-10-3224. Epub 2011 Apr 19. Cancer Res. 2011. PMID: 21505103 Free PMC article.
-
Overcoming kinase resistance in chronic myeloid leukemia.Int J Biochem Cell Biol. 2008;40(3):334-43. doi: 10.1016/j.biocel.2007.10.001. Int J Biochem Cell Biol. 2008. PMID: 18401881 Review.
-
Sequential inhibitor therapy in CML: in vitro simulation elucidates the pattern of resistance mutations after second- and third-line treatment.Clin Cancer Res. 2013 Jun 1;19(11):2962-72. doi: 10.1158/1078-0432.CCR-13-0052. Epub 2013 Apr 2. Clin Cancer Res. 2013. PMID: 23549879
-
New Bcr-Abl inhibitors in chronic myeloid leukemia: keeping resistance in check.Expert Opin Investig Drugs. 2008 Jun;17(6):865-78. doi: 10.1517/13543784.17.6.865. Expert Opin Investig Drugs. 2008. PMID: 18491988 Review.
-
Dynamic change of T315I BCR-ABL kinase domain mutation in Korean chronic myeloid leukaemia patients during treatment with Abl tyrosine kinase inhibitors.Hematol Oncol. 2010 Jun;28(2):82-8. doi: 10.1002/hon.918. Hematol Oncol. 2010. PMID: 19768693
Cited by
-
Cancer Stem Cell Hierarchy in Glioblastoma Multiforme.Front Surg. 2016 Apr 15;3:21. doi: 10.3389/fsurg.2016.00021. eCollection 2016. Front Surg. 2016. PMID: 27148537 Free PMC article. Review.
-
Dasatinib or imatinib in newly diagnosed chronic-phase chronic myeloid leukemia: 2-year follow-up from a randomized phase 3 trial (DASISION).Blood. 2012 Feb 2;119(5):1123-9. doi: 10.1182/blood-2011-08-376087. Epub 2011 Dec 9. Blood. 2012. PMID: 22160483 Free PMC article. Clinical Trial.
-
Perspective: combined forces.Nature. 2013 Jun 27;498(7455):S7. doi: 10.1038/498S7a. Nature. 2013. PMID: 23803949 No abstract available.
-
Chronic myeloid leukemia: advances in understanding disease biology and mechanisms of resistance to tyrosine kinase inhibitors.Curr Hematol Malig Rep. 2015 Jun;10(2):158-66. doi: 10.1007/s11899-015-0248-3. Curr Hematol Malig Rep. 2015. PMID: 25700679 Free PMC article. Review.
-
Chronic Myeloid Leukemia in the Era of Tyrosine Kinase Inhibitors: An Evolving Paradigm of Molecularly Targeted Therapy.Mol Diagn Ther. 2016 Aug;20(4):315-33. doi: 10.1007/s40291-016-0208-1. Mol Diagn Ther. 2016. PMID: 27220498 Review.
References
-
- Schindler T., et al. Structural mechanism for STI-571 inhibition of abelson tyrosine kinase. Science. 2000;289:1938–1942. - PubMed
-
- Shah N.P., et al. Multiple BCR-ABL kinase domain mutations confer polyclonal resistance to the tyrosine kinase inhibitor imatinib (STI571) in chronic phase and blast crisis chronic myeloid leukemia. Cancer Cell. 2002;2:117–125. - PubMed
-
- Shah N.P., et al. Overriding imatinib resistance with a novel ABL kinase inhibitor. Science. 2004;305:399–401. - PubMed
-
- Lombardo L.J., et al. Discovery of N-(2-chloro-6-methyl- phenyl)-2-(6-(4-(2-hydroxyethyl)- piperazin-1-yl)-2-methylpyrimidin-4- ylamino)thiazole-5-carboxamide (BMS-354825), a dual Src/Abl kinase inhibitor with potent antitumor activity in preclinical assays. J. Med. Chem. 2004;47:6658–6661. - PubMed
-
- Tokarski J.S., et al. The structure of dasatinib (BMS-354825) bound to activated ABL kinase domain elucidates its inhibitory activity against imatinib-resistant ABL mutants. Cancer Res. 2006;66:5790–5797. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

