Antihypertensive effects of selective prostaglandin E2 receptor subtype 1 targeting
- PMID: 17710229
- PMCID: PMC1940235
- DOI: 10.1172/JCI29838
Antihypertensive effects of selective prostaglandin E2 receptor subtype 1 targeting
Abstract
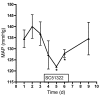
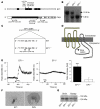
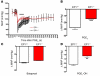
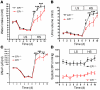
Clinical use of prostaglandin synthase-inhibiting NSAIDs is associated with the development of hypertension; however, the cardiovascular effects of antagonists for individual prostaglandin receptors remain uncharacterized. The present studies were aimed at elucidating the role of prostaglandin E2 (PGE2) E-prostanoid receptor subtype 1 (EP1) in regulating blood pressure. Oral administration of the EP1 receptor antagonist SC51322 reduced blood pressure in spontaneously hypertensive rats. To define whether this antihypertensive effect was caused by EP1 receptor inhibition, an EP1-null mouse was generated using a "hit-and-run" strategy that disrupted the gene encoding EP1 but spared expression of protein kinase N (PKN) encoded at the EP1 locus on the antiparallel DNA strand. Selective genetic disruption of the EP1 receptor blunted the acute pressor response to Ang II and reduced chronic Ang II-driven hypertension. SC51322 blunted the constricting effect of Ang II on in vitro-perfused preglomerular renal arterioles and mesenteric arteriolar rings. Similarly, the pressor response to EP1-selective agonists sulprostone and 17-phenyltrinor PGE2 were blunted by SC51322 and in EP1-null mice. These data support the possibility of targeting the EP1 receptor for antihypertensive therapy.
Figures


Similar articles
-
Activation of prostaglandin E2 EP1 receptor increases arteriolar tone and blood pressure in mice with type 2 diabetes.Cardiovasc Res. 2009 Jul 1;83(1):148-54. doi: 10.1093/cvr/cvp098. Epub 2009 Mar 19. Cardiovasc Res. 2009. PMID: 19299433 Free PMC article.
-
Role of prostaglandin E receptor EP1 subtype in the development of renal injury in genetically hypertensive rats.Hypertension. 2003 Dec;42(6):1183-90. doi: 10.1161/01.HYP.0000101689.64849.97. Hypertension. 2003. PMID: 14670979
-
EP1- and EP3-receptors mediate prostaglandin E2-induced constriction of porcine large cerebral arteries.J Cereb Blood Flow Metab. 2004 Dec;24(12):1305-16. doi: 10.1097/01.WCB.0000139446.61789.14. J Cereb Blood Flow Metab. 2004. PMID: 15625406
-
Prostaglandin E2 modulation of blood pressure homeostasis: studies in rodent models.Prostaglandins Other Lipid Mediat. 2011 Nov;96(1-4):10-3. doi: 10.1016/j.prostaglandins.2011.07.001. Epub 2011 Jul 27. Prostaglandins Other Lipid Mediat. 2011. PMID: 21801847 Free PMC article. Review.
-
EP1 receptor: Devil in emperors coat.J Cell Biochem. 2023 Aug;124(8):1105-1114. doi: 10.1002/jcb.30436. Epub 2023 Jul 14. J Cell Biochem. 2023. PMID: 37450673 Review.
Cited by
-
Endothelial cell prostaglandin E2 receptor EP4 is essential for blood pressure homeostasis.JCI Insight. 2020 Jul 9;5(13):e138505. doi: 10.1172/jci.insight.138505. JCI Insight. 2020. PMID: 32641583 Free PMC article.
-
Central EP3 (E Prostanoid 3) Receptors Mediate Salt-Sensitive Hypertension and Immune Activation.Hypertension. 2019 Dec;74(6):1507-1515. doi: 10.1161/HYPERTENSIONAHA.119.13850. Epub 2019 Nov 4. Hypertension. 2019. PMID: 31679420 Free PMC article.
-
Paradoxical Increase in Mortality and Rupture of Intracranial Aneurysms in Microsomal Prostaglandin E2 Synthase Type 1-Deficient Mice: Attenuation by Aspirin.Neurosurgery. 2015 Oct;77(4):613-20. doi: 10.1227/NEU.0000000000000883. Neurosurgery. 2015. PMID: 26134597 Free PMC article.
-
The prostaglandin EP1 receptor potentiates kainate receptor activation via a protein kinase C pathway and exacerbates status epilepticus.Neurobiol Dis. 2014 Oct;70:74-89. doi: 10.1016/j.nbd.2014.06.004. Epub 2014 Jun 19. Neurobiol Dis. 2014. PMID: 24952362 Free PMC article.
-
Bioactive lipids in hypertension.Adv Pharmacol. 2023;97:1-35. doi: 10.1016/bs.apha.2023.01.001. Epub 2023 May 10. Adv Pharmacol. 2023. PMID: 37236756 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

