Plasmacytoid dendritic cells from mouse tumor-draining lymph nodes directly activate mature Tregs via indoleamine 2,3-dioxygenase
- PMID: 17710230
- PMCID: PMC1940240
- DOI: 10.1172/JCI31911
Plasmacytoid dendritic cells from mouse tumor-draining lymph nodes directly activate mature Tregs via indoleamine 2,3-dioxygenase
Abstract
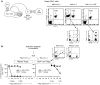
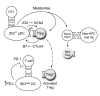
A small population of plasmacytoid DCs (pDCs) in mouse tumor-draining LNs can express the immunoregulatory enzyme indoleamine 2,3-dioxygenase (IDO). We show that these IDO+ pDCs directly activate resting CD4+CD25+Foxp3+ Tregs for potent suppressor activity. In vivo, Tregs isolated from tumor-draining LNs were constitutively activated and suppressed antigen-specific T cells immediately ex vivo. In vitro, IDO+ pDCs from tumor-draining LNs rapidly activated resting Tregs from non-tumor-bearing hosts without the need for mitogen or exogenous anti-CD3 crosslinking. Treg activation by IDO+ pDCs was MHC restricted, required an intact amino acid-responsive GCN2 pathway in the Tregs, and was prevented by CTLA4 blockade. Tregs activated by IDO markedly upregulated programmed cell death 1 ligand 1 (PD-L1) and PD-L2 expression on target DCs, and the ability of Tregs to suppress target T cell proliferation was abrogated by antibodies against the programmed cell death 1/PD-L (PD-1/PD-L) pathway. In contrast, Tregs activated by anti-CD3 crosslinking did not cause upregulation of PD-Ls, and suppression by these cells was unaffected by blocking the PD-1/PD-L pathway. Tregs isolated from tumor-draining LNs in vivo showed potent PD-1/PD-L-mediated suppression, which was selectively lost when tumors were grown in IDO-deficient hosts. We hypothesize that IDO+ pDCs create a profoundly suppressive microenvironment within tumor-draining LNs via constitutive activation of Tregs.
Figures



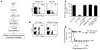


References
-
- Munn D.H., et al. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 1998;281:1191–1193. - PubMed
-
- Gurtner G.J., et al. Inhibition of indoleamine 2,3-dioxygenase augments trinitrobenzene sulfonic acid colitis in mice. Gastroenterology. 2003;125:1762–1773. - PubMed
-
- Liu H., Liu L., Fletcher B.S., Visner G.A. Sleeping Beauty-based gene therapy with indoleamine 2,3-dioxygenase inhibits lung allograft fibrosis. FASEB J. 2006;20:2384–2386. - PubMed
-
- Uyttenhove C., et al. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat. Med. 2003;9:1269–1274. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

