Toll-like receptor 2 contributes to antibacterial defence against pneumolysin-deficient pneumococci
- PMID: 17711480
- PMCID: PMC2253695
- DOI: 10.1111/j.1462-5822.2007.01035.x
Toll-like receptor 2 contributes to antibacterial defence against pneumolysin-deficient pneumococci
Abstract
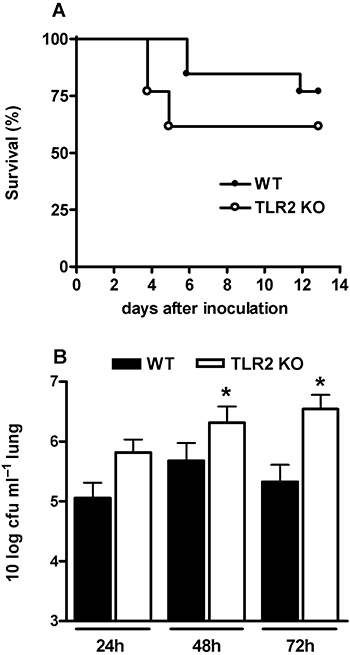
Toll-like receptors (TLRs) are pattern recognition receptors that recognize conserved molecular patterns expressed by pathogens. Pneumolysin, an intracellular toxin found in all Streptococcus pneumoniae clinical isolates, is an important virulence factor of the pneumococcus that is recognized by TLR4. Although TLR2 is considered the most important receptor for Gram-positive bacteria, our laboratory previously could not demonstrate a decisive role for TLR2 in host defence against pneumonia caused by a serotype 3 S. pneumoniae. Here we tested the hypothesis that in the absence of TLR2, S. pneumoniae can still be sensed by the immune system through an interaction between pneumolysin and TLR4. C57BL/6 wild-type (WT) and TLR2 knockout (KO) mice were intranasally infected with either WT S. pneumoniae D39 (serotype 2) or the isogenic pneumolysin-deficient S. pneumoniae strain D39 PLN. TLR2 did not contribute to antibacterial defence against WT S. pneumoniae D39. In contrast, pneumolysin-deficient S. pneumoniae only grew in lungs of TLR2 KO mice. TLR2 KO mice displayed a strongly reduced early inflammatory response in their lungs during pneumonia caused by both pneumolysin-producing and pneumolysin-deficient pneumococci. These data suggest that pneumolysin-induced TLR4 signalling can compensate for TLR2 deficiency during respiratory tract infection with S. pneumoniae.
Figures



Similar articles
-
Role played by Toll-like receptors 2 and 4 in lipoteichoic acid-induced lung inflammation and coagulation.J Infect Dis. 2008 Jan 15;197(2):245-52. doi: 10.1086/524873. J Infect Dis. 2008. PMID: 18179383
-
Innate immunity to pneumococcal infection of the central nervous system depends on toll-like receptor (TLR) 2 and TLR4.J Infect Dis. 2008 Oct 1;198(7):1028-36. doi: 10.1086/591626. J Infect Dis. 2008. PMID: 18700834
-
Toll-like receptor 2 does not contribute to host response during postinfluenza pneumococcal pneumonia.Am J Respir Cell Mol Biol. 2007 May;36(5):609-14. doi: 10.1165/rcmb.2006-0166OC. Epub 2006 Dec 14. Am J Respir Cell Mol Biol. 2007. PMID: 17170383
-
Toll-like receptor-dependent discrimination of streptococci.J Endotoxin Res. 2006;12(5):307-12. doi: 10.1179/096805106X118762. J Endotoxin Res. 2006. PMID: 17059694 Review.
-
Of mice and men: innate immunity in pneumococcal pneumonia.Int J Antimicrob Agents. 2010 Feb;35(2):107-13. doi: 10.1016/j.ijantimicag.2009.10.002. Epub 2009 Dec 14. Int J Antimicrob Agents. 2010. PMID: 20005681 Review.
Cited by
-
Streptococcal co-infection augments Candida pathogenicity by amplifying the mucosal inflammatory response.Cell Microbiol. 2014 Feb;16(2):214-31. doi: 10.1111/cmi.12216. Epub 2013 Oct 17. Cell Microbiol. 2014. PMID: 24079976 Free PMC article.
-
Role of phosphoinositide 3-kinase-Akt signaling pathway in the age-related cytokine dysregulation in splenic macrophages stimulated via TLR-2 or TLR-4 receptors.Mech Ageing Dev. 2011 Jun-Jul;132(6-7):274-86. doi: 10.1016/j.mad.2011.05.003. Epub 2011 May 27. Mech Ageing Dev. 2011. PMID: 21645538 Free PMC article.
-
TLR-mediated inflammatory responses to Streptococcus pneumoniae are highly dependent on surface expression of bacterial lipoproteins.J Immunol. 2014 Oct 1;193(7):3736-45. doi: 10.4049/jimmunol.1401413. Epub 2014 Aug 29. J Immunol. 2014. PMID: 25172490 Free PMC article. Clinical Trial.
-
The role of TLR2 in the host response to pneumococcal pneumonia in absence of the spleen.BMC Infect Dis. 2012 Jun 21;12:139. doi: 10.1186/1471-2334-12-139. BMC Infect Dis. 2012. PMID: 22721450 Free PMC article.
-
Double deficiency of toll-like receptors 2 and 4 alters long-term neurological sequelae in mice cured of pneumococcal meningitis.Sci Rep. 2019 Nov 7;9(1):16189. doi: 10.1038/s41598-019-52212-7. Sci Rep. 2019. PMID: 31700009 Free PMC article.
References
-
- Albiger B, Sandgren A, Katsuragi H, Meyer-Hoffert U, Beiter K, Wartha F, et al. Myeloid differentiation factor 88-dependent signalling controls bacterial growth during colonization and systemic pneumococcal disease in mice. Cell Microbiol. 2005;7:1603–1615. - PubMed
-
- Alouf JE. Cholesterol-binding cytolytic protein toxins. Int J Med Microbiol. 2000;290:351–356. - PubMed
-
- Bernstein JM. Treatment of community-acquired pneumonia – IDSA guidelines. Infectious Diseases Society of America. Chest. 1999;115:9S–13S. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

