Exposure-response analysis reveals that clinically important toxicity difference can exist between bioequivalent carbamazepine tablets
- PMID: 17711537
- PMCID: PMC2291269
- DOI: 10.1111/j.1365-2125.2007.02984.x
Exposure-response analysis reveals that clinically important toxicity difference can exist between bioequivalent carbamazepine tablets
Abstract
Aims: To assess whether, using the current regulatory criteria, therapeutically important differences can exist between bioequivalent carbamazepine (CBZ) tablets. A secondary goal was to demonstrate quantitatively the relationship between the risk of neurological adverse effects to orally ingested CBZ and the rate of absorption.
Methods: Results of a bioequivalence study by Olling et al. (Biopharm Drug Dispos 1999; 20: 19-28) were reanalysed. Following an exploratory data analysis step, a mixed-effect pharmacokinetic-pharmacodynamic (PK-PD) model was built to describe the dependence of adverse events on the CBZ concentration.
Results: Rapid development of tolerance was demonstrated for most neurological adverse effects, with a characteristic half-life of 02.29 h and an initial EC50 of 2.33 mg l(-1). The resulting tolerance PK-PD model was characterized further using the tools and terminology of sensitivity analysis. It was demonstrated that the maximum concentration (C(max)) exhibits poor PK and PD sensitivities, and that clinically significant differences can exist between formulations which otherwise comply with the bioequivalence requirements. In contrast, another PK metric, the partial AUC, was a much better marker of the early neurological adverse events observable during the absorption phase of the drug.
Conclusions: In clinical and regulatory considerations, the development of acute tolerance for adverse effects of CBZ must be taken into account. Partial AUC reflects more sensitively the risk of adverse events than C(max). Instead of the current trend of tightening of the bioequivalence criteria for narrow therapeutic index drugs, the use of alternative, more sensitive PK metrics is proposed.
Figures
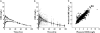



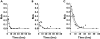

 ); C′, (▾); D′, (□)). The concentration profiles were simulated by assuming common distribution (Vd/F) and elimination (CL) parameters, but different absorption rate constants (ka). The actual numerical parameters can be found in Table 1: CL and Vd/F, product D; ka values, products A, B, C and D, respectively. (B) Predicted probabilities of observing adverse events with the hypothetical A′ (•); B′ (
); C′, (▾); D′, (□)). The concentration profiles were simulated by assuming common distribution (Vd/F) and elimination (CL) parameters, but different absorption rate constants (ka). The actual numerical parameters can be found in Table 1: CL and Vd/F, product D; ka values, products A, B, C and D, respectively. (B) Predicted probabilities of observing adverse events with the hypothetical A′ (•); B′ ( ); C′ (▾); and the reference D′ (□) formulations. (C) Ratios of Cmax, AUCP and Rmax relative to the reference formulation. A′, (▪); B′, (
); C′ (▾); and the reference D′ (□) formulations. (C) Ratios of Cmax, AUCP and Rmax relative to the reference formulation. A′, (▪); B′, ( ); C′, (
); C′, ( ); D′, (
); D′, ( )
)
Similar articles
-
Existing and new criteria for bioequivalence evaluation of new controlled release (CR) products of carbamazepine.Epilepsy Res. 1998 Nov;32(3):371-8. doi: 10.1016/s0920-1211(98)00064-3. Epilepsy Res. 1998. PMID: 9839777 Clinical Trial.
-
Pharmacokinetic interaction study between the new antiepileptic and CNS drug RWJ-333369 and carbamazepine in healthy adults.Epilepsia. 2006 Nov;47(11):1830-40. doi: 10.1111/j.1528-1167.2006.00815.x. Epilepsia. 2006. PMID: 17116022 Clinical Trial.
-
A single-dose, three-period, six-sequence crossover study comparing the bioavailability of solution, suspension, and enteric-coated tablets of magnesium valproate in healthy Mexican volunteers under fasting conditions.Clin Ther. 2009 Sep;31(9):2002-11. doi: 10.1016/j.clinthera.2009.09.016. Clin Ther. 2009. PMID: 19843490 Clinical Trial.
-
Bioequivalence of a single 10-mg dose of finasteride 5-mg oral disintegrating tablets and standard tablets in healthy adult male Han Chinese volunteers: a randomized sequence, open-label, two-way crossover study.Clin Ther. 2009 Oct;31(10):2242-8. doi: 10.1016/j.clinthera.2009.09.015. Clin Ther. 2009. PMID: 19922895 Clinical Trial.
-
Assessment of the bioequivalence of two formulations of clarithromycin extended-release 500-mg tablets under fasting and fed conditions: a single-dose, randomized, open-label, two-period, two-way crossover study in healthy Jordanian male volunteers.Clin Ther. 2008 Oct;30(10):1831-43. doi: 10.1016/j.clinthera.2008.10.010. Clin Ther. 2008. PMID: 19014838 Clinical Trial.
Cited by
-
Evaluation of bioequivalence for highly variable drugs with scaled average bioequivalence.Clin Pharmacokinet. 2009;48(11):725-43. doi: 10.2165/11318040-000000000-00000. Clin Pharmacokinet. 2009. PMID: 19817502 Review.
-
Generic products of antiepileptic drugs: a perspective on bioequivalence, bioavailability, and formulation switches using Monte Carlo simulations.CNS Drugs. 2014 Jan;28(1):69-77. doi: 10.1007/s40263-013-0112-8. CNS Drugs. 2014. PMID: 24092569
-
Looking back: editors' pick of 2008.Br J Clin Pharmacol. 2009 Jan;67(1):1-4. doi: 10.1111/j.1365-2125.2008.03354.x. Br J Clin Pharmacol. 2009. PMID: 19133056 Free PMC article. No abstract available.
-
Metrics for the evaluation of bioequivalence of modified-release formulations.AAPS J. 2012 Dec;14(4):813-9. doi: 10.1208/s12248-012-9396-8. Epub 2012 Aug 22. AAPS J. 2012. PMID: 22910857 Free PMC article. Review.
-
Clinical utility of eslicarbazepine: current evidence.Drug Des Devel Ther. 2015 Feb 10;9:781-9. doi: 10.2147/DDDT.S57409. eCollection 2015. Drug Des Devel Ther. 2015. PMID: 25709402 Free PMC article. Review.
References
-
- Besag FM. Is generic prescribing acceptable in epilepsy? Drug Safety. 2000;23:173–82. - PubMed
-
- Crawford P, Hall WW, Chappell B, Collings J, Stewart A. Generic prescribing for epilepsy. Is it safe? Seizure. 1996;5:1–5. - PubMed
-
- Neuvonen PJ. Bioavailability and central side effects of different carbamazepine tablets. Int J Clin Pharmacol Ther Toxicol. 1985;23:226–32. - PubMed
-
- Wolf P, May T, Tiska G, Schreiber G. Steady state concentrations and diurnal fluctuations of carbamazepine in patients after different slow release formulations. Arzneimittelforschung. 1992;42:284–8. - PubMed
-
- Hoppener RJ, Kuyer A, Meijer JW, Hulsman J. Correlation between daily fluctuations of carbamazepine serum levels and intermittent side effects. Epilepsia. 1980;21:341–50. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

