Glucocorticoid modulation of insulin signaling in human subcutaneous adipose tissue
- PMID: 17711920
- PMCID: PMC7611650
- DOI: 10.1210/jc.2007-1399
Glucocorticoid modulation of insulin signaling in human subcutaneous adipose tissue
Abstract
Context: Glucocorticoid (GC) excess is characterized by central obesity, insulin resistance, and in some cases, type 2 diabetes. However, the impact of GC upon insulin signaling in human adipose tissue has not been fully explored.
Objective: We have examined the effect of GC upon insulin signaling in both human sc primary preadipocyte cultures and a novel human immortalized sc adipocyte cell line (Chub-S7) and contrasted this with observations in primary cultures of human skeletal muscle.
Design and setting: This is an in vitro study characterizing the impact of GC upon insulin signaling in human tissues.
Patients: Biopsy specimens were from healthy volunteers who gave their full and informed written consent.
Interventions: Combinations of treatments, including GC, RU38486, and wortmannin, were used.
Main outcome measures: Insulin signaling cascade gene and protein expression and insulin-stimulated glucose uptake were determined.
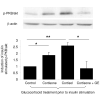
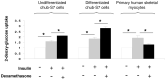
Results: In human adipocytes, pretreatment with GC induced a dose-dependent [1.0 (control); 1.2 +/- 0.1 (50 nm); 2.2 +/- 0.2 (250 nm), P < 0.01 vs. control; 3.4 +/- 0.2 (1000 nm), P < 0.001 vs. control] and time-dependent [1.0 (1 h); 3.2 +/- 2.0 (6 h); 9.1 +/- 5.9 (24 h), P < 0.05 vs. 1 h; 4.5 +/- 2.2 (48 h)] increase in insulin-stimulated protein kinase B/akt phosphorylation. In addition, whereas insulin receptor substrate (IRS)-1 protein expression did not change, IRS-1 tyrosine phosphorylation increased. Furthermore, GC induced IRS-2 mRNA expression (2.8-fold; P < 0.05) and increased insulin-stimulated glucose uptake [1.0 (control) 1.8 +/- 0.1 (insulin) vs. 2.8 +/- 0.2 (insulin + GC); P < 0.05]. In contrast, in primary cultures of human muscle, GC decreased insulin-stimulated glucose uptake [1.0 (control) 1.9 +/- 0.2 (insulin) vs. GC 1.3 +/- 0.1 (insulin + GC); P < 0.05].
Conclusions: We have demonstrated tissue-specific regulation of insulin signaling by GC. Within sc adipose tissue, GCs augment insulin signaling, yet in muscle GCs cause insulin resistance. We propose that enhanced insulin action in adipose tissue increases adipocyte differentiation, thereby contributing to GC-induced obesity.
Figures




References
-
- Carey DG, Jenkins AB, Campbell LV, Freund J, Chisholm DJ. Abdominal fat and insulin resistance in normal and overweight women: Direct measurements reveal a strong relationship in subjects at both low and high risk of NIDDM. Diabetes. 1996;45(5):633–638. - PubMed
-
- Fontbonne A, Thibult N, Eschwege E, Ducimetiere P. Body fat distribution and coronary heart disease mortality in subjects with impaired glucose tolerance or diabetes mellitus: the Paris Prospective Study, 15-year follow-up. Diabetologia. 1992;35(5):464–468. - PubMed
-
- Van Staa TP, Leufkens HG, Abenhaim L, Begaud B, Zhang B, Cooper C. Use of oral corticosteroids in the United Kingdom. QJM. 2000;93(2):105–111. - PubMed
-
- Strack AM, Sebastian RJ, Schwartz MW, Dallman MF. Glucocorticoids and insulin: reciprocal signals for energy balance. Am J Physiol. 1995;268(1 Pt 2):R142–R149. - PubMed
-
- Schneiter P, Tappy L. Kinetics of dexamethasone-induced alterations of glucose metabolism in healthy humans. Am J Physiol. 1998;275(5 Pt 1):E806–E813. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

