Cellular immune responses induced with dose-sparing intradermal administration of HIV vaccine to HIV-uninfected volunteers in the ANRS VAC16 trial
- PMID: 17712402
- PMCID: PMC1942115
- DOI: 10.1371/journal.pone.0000725
Cellular immune responses induced with dose-sparing intradermal administration of HIV vaccine to HIV-uninfected volunteers in the ANRS VAC16 trial
Abstract
Objective: The objective was to compare the safety and cellular immunogenicity of intradermal versus intramuscular immunization with an HIV-lipopeptide candidate vaccine (LIPO-4) in healthy volunteers.
Methodology: A randomized, open-label trial with 24 weeks of follow-up was conducted in France at six HIV-vaccine trial sites. Sixty-eight healthy 21- to 55-year-old HIV-uninfected subjects were randomized to receive the LIPO-4 vaccine (four HIV lipopeptides linked to a T-helper-stimulating epitope of tetanus-toxin protein) at weeks 0, 4 and 12, either intradermally (0.1 ml, 100 microg of each peptide) or intramuscularly (0.5 ml, 500 microg of each peptide). Comparative safety of both routes was evaluated. CD8+ T-cell immune responses to HIV epitopes (ELISpot interferon-gamma assay) and tetanus toxin-specific CD4+ T-cell responses (lymphoproliferation) were assessed at baseline, two weeks after each injection, and at week 24.
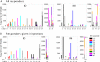
Results and conclusion: No severe, serious or life-threatening adverse events were observed. Local pain was significantly more frequent after intramuscular injection, but local inflammatory reactions were more frequent after intradermal immunization. At weeks 2, 6, 14 and 24, the respective cumulative percentages of induced CD8+ T-cell responses to at least one HIV peptide were 9, 33, 39 and 52 (intradermal group) or 14, 20, 26 and 37 (intramuscular group), and induced tetanus toxin-specific CD4+ T-cell responses were 6, 27, 33 and 39 (intradermal), or 9, 46, 54 and 63 (intramuscular). In conclusion, intradermal LIPO-4 immunization was well tolerated, required one-fifth of the intramuscular dose, and induced similar HIV-specific CD8+ T-cell responses. Moreover, the immunization route influenced which antigen-specific T-cells (CD4+ or CD8+) were induced.
Trial registration: ClinicalTrials.gov NCT00121121.
Conflict of interest statement
Figures

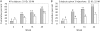

Similar articles
-
Long-term CD4(+) and CD8(+) T-cell responses induced in HIV-uninfected volunteers following intradermal or intramuscular administration of an HIV-lipopeptide vaccine (ANRS VAC16).Vaccine. 2013 Sep 13;31(40):4406-15. doi: 10.1016/j.vaccine.2013.06.102. Epub 2013 Jul 11. Vaccine. 2013. PMID: 23850610 Clinical Trial.
-
Immunogenicity and safety of an HIV-1 lipopeptide vaccine in healthy adults: a phase 2 placebo-controlled ANRS trial.AIDS. 2010 Sep 10;24(14):2211-23. doi: 10.1097/QAD.0b013e32833ce566. AIDS. 2010. PMID: 20625264 Clinical Trial.
-
Phase I safety and immunogenicity evaluation of MVA-CMDR, a multigenic, recombinant modified vaccinia Ankara-HIV-1 vaccine candidate.PLoS One. 2010 Nov 15;5(11):e13983. doi: 10.1371/journal.pone.0013983. PLoS One. 2010. PMID: 21085591 Free PMC article. Clinical Trial.
-
Intradermal vaccination of HIV-infected patients with short HIV Gag p24-like peptides induces CD4 + and CD8 + T cell responses lasting more than seven years.Scand J Infect Dis. 2012 Aug;44(8):566-72. doi: 10.3109/00365548.2011.653581. Epub 2012 Feb 19. Scand J Infect Dis. 2012. PMID: 22339485 Clinical Trial.
-
Vaccine applications of flow cytometry.Methods. 2012 Jul;57(3):383-91. doi: 10.1016/j.ymeth.2012.01.001. Epub 2012 Jan 10. Methods. 2012. PMID: 22251671 Free PMC article. Review.
Cited by
-
Accelerating clinical development of HIV vaccine strategies: methodological challenges and considerations in constructing an optimised multi-arm phase I/II trial design.Trials. 2014 Feb 26;15:68. doi: 10.1186/1745-6215-15-68. Trials. 2014. PMID: 24571662 Free PMC article.
-
New concepts in herpes simplex virus vaccine development: notes from the battlefield.Expert Rev Vaccines. 2009 Aug;8(8):1023-35. doi: 10.1586/erv.09.60. Expert Rev Vaccines. 2009. PMID: 19627185 Free PMC article. Review.
-
Design of multi-epitope peptides containing HLA class-I and class-II-restricted epitopes derived from immunogenic Leishmania proteins, and evaluation of CD4+ and CD8+ T cell responses induced in cured cutaneous leishmaniasis subjects.PLoS Negl Trop Dis. 2020 Mar 16;14(3):e0008093. doi: 10.1371/journal.pntd.0008093. eCollection 2020 Mar. PLoS Negl Trop Dis. 2020. PMID: 32176691 Free PMC article.
-
TLR-based immune adjuvants.Vaccine. 2011 Apr 12;29(17):3341-55. doi: 10.1016/j.vaccine.2010.08.002. Epub 2010 Aug 14. Vaccine. 2011. PMID: 20713100 Free PMC article. Review.
-
Novel directions in HIV-1 vaccines revealed from clinical trials.Curr Opin HIV AIDS. 2013 Sep;8(5):421-31. doi: 10.1097/COH.0b013e3283632c26. Curr Opin HIV AIDS. 2013. PMID: 23743791 Free PMC article. Review.
References
-
- Markel H. The search for effective HIV vaccines. N Engl J Med. 2005;353:753–757. - PubMed
-
- Klinguer C, David D, Kouach M, Wieruszeski JM, Tartar A, et al. Characterization of a multi-lipopeptides mixture used as an HIV-1 vaccine candidate. Vaccine. 1999;18:259–267. - PubMed
-
- Deres K, Schild H, Wiesmuller KH, Jung G, Rammensee HG. In vivo priming of virus-specific cytotoxic T lymphocytes with synthetic lipopeptide vaccine. Nature. 1989;342:561–564. - PubMed
-
- Martinon F, Gras-Masse H, Boutillon C, Chirat F, Deprez B, et al. Immunization of mice with lipopeptides bypasses the prerequisite for adjuvant. Immune response of BALB/c mice to human immunodeficiency virus envelope glycoprotein. J Immunol. 1992;149:3416–3422. - PubMed
-
- Sauzet JP, Deprez B, Martinon F, Guillet JG, Gras-Masse H, Gomard E. Long-lasting anti-viral cytotoxic T lymphocytes induced in vivo with chimeric-multirestricted lipopeptides. Vaccine. 1995;13:1339–1345. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

