High cooperativity of the SV40 major capsid protein VP1 in virus assembly
- PMID: 17712413
- PMCID: PMC1942081
- DOI: 10.1371/journal.pone.0000765
High cooperativity of the SV40 major capsid protein VP1 in virus assembly
Abstract
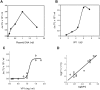
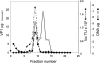
SV40 is a small, non enveloped DNA virus with an icosahedral capsid of 45 nm. The outer shell is composed of pentamers of the major capsid protein, VP1, linked via their flexible carboxy-terminal arms. Its morphogenesis occurs by assembly of capsomers around the viral minichromosome. However the steps leading to the formation of mature virus are poorly understood. Intermediates of the assembly reaction could not be isolated from cells infected with wt SV40. Here we have used recombinant VP1 produced in insect cells for in vitro assembly studies around supercoiled heterologous plasmid DNA carrying a reporter gene. This strategy yields infective nanoparticles, affording a simple quantitative transduction assay. We show that VP1 assembles under physiological conditions into uniform nanoparticles of the same shape, size and CsCl density as the wild type virus. The stoichiometry is one DNA molecule per capsid. VP1 deleted in the C-arm, which is unable to assemble but can bind DNA, was inactive indicating genuine assembly rather than non-specific DNA-binding. The reaction requires host enzymatic activities, consistent with the participation of chaperones, as recently shown. Our results demonstrate dramatic cooperativity of VP1, with a Hill coefficient of approximately 6. These findings suggest that assembly may be a concerted reaction. We propose that concerted assembly is facilitated by simultaneous binding of multiple capsomers to a single DNA molecule, as we have recently reported, thus increasing their local concentration. Emerging principles of SV40 assembly may help understanding assembly of other complex systems. In addition, the SV40-based nanoparticles described here are potential gene therapy vectors that combine efficient gene delivery with safety and flexibility.
Conflict of interest statement
Figures

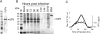
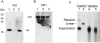

Similar articles
-
Assemblages of simian virus 40 capsid proteins and viral DNA visualized by electron microscopy.Biochem Biophys Res Commun. 2007 Feb 9;353(2):424-30. doi: 10.1016/j.bbrc.2006.12.038. Epub 2006 Dec 13. Biochem Biophys Res Commun. 2007. PMID: 17189615
-
Cellular transcription factor Sp1 recruits simian virus 40 capsid proteins to the viral packaging signal, ses.J Virol. 2002 Jun;76(12):5915-24. doi: 10.1128/jvi.76.12.5915-5924.2002. J Virol. 2002. PMID: 12021324 Free PMC article.
-
Simian virus 40 VP1 capsid protein forms polymorphic assemblies in vitro.J Gen Virol. 2003 Jul;84(Pt 7):1899-1905. doi: 10.1099/vir.0.19067-0. J Gen Virol. 2003. PMID: 12810885
-
Simian virus 40 chromatin interaction with the capsid proteins.J Biomol Struct Dyn. 1983 Dec;1(3):689-704. doi: 10.1080/07391102.1983.10507475. J Biomol Struct Dyn. 1983. PMID: 6101085 Review.
-
Host range analysis of simian virus 40, BK virus and chimaeric SV40/BKV: relative expression of large T-antigen and Vp1 in infected and transformed cells.Dev Biol Stand. 1998;94:191-205. Dev Biol Stand. 1998. PMID: 9776240 Review.
Cited by
-
Downregulation of the stress-induced ligand ULBP1 following SV40 infection confers viral evasion from NK cell cytotoxicity.Oncotarget. 2016 Mar 29;7(13):15369-81. doi: 10.18632/oncotarget.8085. Oncotarget. 2016. PMID: 26992229 Free PMC article.
-
Quantum dot-induced viral capsid assembling in dissociation buffer.Int J Nanomedicine. 2013;8:2119-28. doi: 10.2147/IJN.S44534. Epub 2013 Jun 5. Int J Nanomedicine. 2013. PMID: 23776332 Free PMC article.
-
Effect of capsid confinement on the chromatin organization of the SV40 minichromosome.Nucleic Acids Res. 2013 Feb 1;41(3):1569-80. doi: 10.1093/nar/gks1270. Epub 2012 Dec 20. Nucleic Acids Res. 2013. PMID: 23258701 Free PMC article.
-
Revealing in real-time a multistep assembly mechanism for SV40 virus-like particles.Sci Adv. 2020 Apr 15;6(16):eaaz1639. doi: 10.1126/sciadv.aaz1639. eCollection 2020 Apr. Sci Adv. 2020. PMID: 32494611 Free PMC article.
-
Effect of ionic strength on the assembly of simian vacuolating virus capsid protein around poly(styrene sulfonate).Eur Phys J E Soft Matter. 2023 Nov 2;46(11):107. doi: 10.1140/epje/s10189-023-00363-x. Eur Phys J E Soft Matter. 2023. PMID: 37917241 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

