Condensin I reveals new insights on mouse meiotic chromosome structure and dynamics
- PMID: 17712430
- PMCID: PMC1942118
- DOI: 10.1371/journal.pone.0000783
Condensin I reveals new insights on mouse meiotic chromosome structure and dynamics
Abstract
Chromosome shaping and individualization are necessary requisites to warrant the correct segregation of genomes in either mitotic or meiotic cell divisions. These processes are mainly prompted in vertebrates by three multiprotein complexes termed cohesin and condensin I and II. In the present study we have analyzed by immunostaining the appearance and subcellular distribution of condensin I in mouse mitotic and meiotic chromosomes. Our results demonstrate that in either mitotically or meiotically dividing cells, condensin I is loaded onto chromosomes by prometaphase. Condensin I is detectable as a fuzzy axial structure running inside chromatids of condensed chromosomes. The distribution of condensin I along the chromosome length is not uniform, since it preferentially accumulates close to the chromosome ends. Interestingly, these round accumulations found at the condensin I axes termini colocalized with telomere complexes. Additionally, we present the relative distribution of the condensin I and cohesin complexes in metaphase I bivalents. All these new data have allowed us to propose a comprehensive model for meiotic chromosome structure.
Conflict of interest statement
Figures
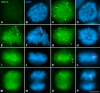
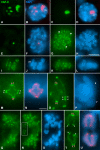
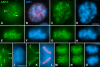

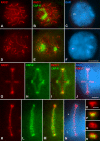
Similar articles
-
Contribution of hCAP-D2, a non-SMC subunit of condensin I, to chromosome and chromosomal protein dynamics during mitosis.Mol Cell Biol. 2005 Jan;25(2):740-50. doi: 10.1128/MCB.25.2.740-750.2005. Mol Cell Biol. 2005. PMID: 15632074 Free PMC article.
-
Aurora B controls the association of condensin I but not condensin II with mitotic chromosomes.J Cell Sci. 2007 Apr 1;120(Pt 7):1245-55. doi: 10.1242/jcs.03425. Epub 2007 Mar 13. J Cell Sci. 2007. PMID: 17356064
-
Condensin restructures chromosomes in preparation for meiotic divisions.J Cell Biol. 2004 Nov 22;167(4):613-25. doi: 10.1083/jcb.200408061. J Cell Biol. 2004. PMID: 15557118 Free PMC article.
-
Chromosome dynamics: the case of the missing condensin.Curr Biol. 2009 Feb 10;19(3):R127-9. doi: 10.1016/j.cub.2008.12.004. Curr Biol. 2009. PMID: 19211053 Review.
-
Meiotic chromosome structure and function in plants.Cytogenet Genome Res. 2014;143(1-3):6-17. doi: 10.1159/000365260. Epub 2014 Jul 30. Cytogenet Genome Res. 2014. PMID: 25096046 Review.
Cited by
-
Condensins: universal organizers of chromosomes with diverse functions.Genes Dev. 2012 Aug 1;26(15):1659-78. doi: 10.1101/gad.194746.112. Genes Dev. 2012. PMID: 22855829 Free PMC article. Review.
-
Dynamics of cohesin subunits in grasshopper meiotic divisions.Chromosoma. 2013 Mar;122(1-2):77-91. doi: 10.1007/s00412-012-0393-6. Epub 2013 Jan 4. Chromosoma. 2013. PMID: 23283389
-
Kinase PLK1 regulates the disassembly of the lateral elements and the assembly of the inner centromere during the diakinesis/metaphase I transition in male mouse meiosis.Front Cell Dev Biol. 2023 Jan 13;10:1069946. doi: 10.3389/fcell.2022.1069946. eCollection 2022. Front Cell Dev Biol. 2023. PMID: 36733339 Free PMC article.
-
MiR-1976/NCAPH/P65 axis inhibits the malignant phenotypes of lung adenocarcinoma.Sci Rep. 2024 May 16;14(1):11211. doi: 10.1038/s41598-024-61261-6. Sci Rep. 2024. PMID: 38755247 Free PMC article.
-
Roles of cohesin and condensin in chromosome dynamics during mammalian meiosis.J Reprod Dev. 2013 Oct;59(5):431-6. doi: 10.1262/jrd.2013-068. J Reprod Dev. 2013. PMID: 24162807 Free PMC article. Review.
References
-
- Swedlow JR, Hirano T. The making of the mitotic chromosome: modern insights into classical questions. Mol Cell. 2003;11:557–69. - PubMed
-
- Hirano T. Condensins: organizing and segregating the genome. Curr Biol. 2005;15:R265–75. - PubMed
-
- Belmont AS. Mitotic chromosome structure and condensation. Curr Opin Cell Biol. 2006;18:632–8. - PubMed
-
- Losada A, Hirano T. Dynamic molecular linkers of the genome: the first decade of SMC proteins. Genes Dev. 2005;19:1269–87. - PubMed
-
- Nasmyth K, Haering CH. The structure and function of SMC and kleisin complexes. Annu Rev Biochem. 2005;74:595–648. - PubMed

