Estrogen receptor genotypes, menopausal status, and the lipid effects of tamoxifen
- PMID: 17713466
- PMCID: PMC2782693
- DOI: 10.1038/sj.clpt.6100343
Estrogen receptor genotypes, menopausal status, and the lipid effects of tamoxifen
Abstract
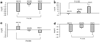
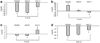
Tamoxifen induces important changes in serum lipid profiles in some women; however, little information is available to predict which women will experience improved lipid profiles during tamoxifen therapy. As part of a multicenter prospective observational trial in 176 breast cancer patients, we tested the hypothesis that tamoxifen-induced lipid changes were associated with genetic variants in candidate target genes (CYP2D6, ESR1, and ESR2). Tamoxifen lowered low-density lipoprotein cholesterol (P<0.0001) by 23.5 mg/dl (13.5-33.5 mg/dl) and increased triglycerides (P=0.006). In postmenopausal women, the ESR1-XbaI and ESR2-02 genotypes were associated with tamoxifen-induced changes in total cholesterol (P=0.03; GG vs GA/AA) and triglycerides (P=0.01; gene-dose effect), respectively. In premenopausal women, the ESR1-XbaI genotypes were associated with tamoxifen-induced changes in triglycerides (P=0.002; gene-dose effect) and high-density lipoprotein (P=0.004; gene-dose effect). Our results suggest that estrogen receptor genotyping may be useful in predicting which women would benefit more from tamoxifen.
Trial registration: ClinicalTrials.gov NCT00228930.
Conflict of interest statement
The authors declared no conflict of interest.
Figures

Similar articles
-
Estrogen receptor genotypes, menopausal status, and the effects of tamoxifen on lipid levels: revised and updated results.Clin Pharmacol Ther. 2010 Nov;88(5):626-9. doi: 10.1038/clpt.2010.143. Epub 2010 Sep 8. Clin Pharmacol Ther. 2010. PMID: 20827267 Free PMC article. Clinical Trial.
-
ESR1 and ESR2 polymorphisms in the BIG 1-98 trial comparing adjuvant letrozole versus tamoxifen or their sequence for early breast cancer.Breast Cancer Res Treat. 2015 Dec;154(3):543-55. doi: 10.1007/s10549-015-3634-6. Epub 2015 Nov 21. Breast Cancer Res Treat. 2015. PMID: 26590813 Free PMC article. Clinical Trial.
-
Lack of association between oestrogen receptor polymorphisms and change in bone mineral density with tamoxifen therapy.Br J Cancer. 2010 Jan 19;102(2):294-300. doi: 10.1038/sj.bjc.6605460. Epub 2009 Dec 1. Br J Cancer. 2010. PMID: 19953095 Free PMC article. Clinical Trial.
-
Tamoxifen, cytochrome P450 genes and breast cancer clinical outcomes.Breast. 2011 Apr;20(2):111-8. doi: 10.1016/j.breast.2010.11.003. Epub 2010 Dec 24. Breast. 2011. PMID: 21185724 Review.
-
Comparative assessment of lipid effects of endocrine therapy for breast cancer: implications for cardiovascular disease prevention in postmenopausal women.Breast. 2006 Jun;15(3):301-12. doi: 10.1016/j.breast.2005.08.033. Epub 2005 Oct 17. Breast. 2006. PMID: 16230014 Review.
Cited by
-
Estrogen receptor genotypes, menopausal status, and the effects of tamoxifen on lipid levels: revised and updated results.Clin Pharmacol Ther. 2010 Nov;88(5):626-9. doi: 10.1038/clpt.2010.143. Epub 2010 Sep 8. Clin Pharmacol Ther. 2010. PMID: 20827267 Free PMC article. Clinical Trial.
-
Serum Cholesterol Level Changes during Gonadotropin-Releasing Hormone-Agonist Therapy in Premenopausal Female Patients with Breast Cancer.J Menopausal Med. 2024 Aug;30(2):120-125. doi: 10.6118/jmm.24009. J Menopausal Med. 2024. PMID: 39315503 Free PMC article.
-
Estrogen receptor genotypes influence hot flash prevalence and composite score before and after tamoxifen therapy.J Clin Oncol. 2008 Dec 20;26(36):5849-54. doi: 10.1200/JCO.2008.16.8377. Epub 2008 Nov 17. J Clin Oncol. 2008. PMID: 19018086 Free PMC article.
-
Comparison of changes in the lipid profile of postmenopausal women with early stage breast cancer treated with exemestane or letrozole.J Clin Pharmacol. 2012 Dec;52(12):1852-60. doi: 10.1177/0091270011424153. Epub 2011 Dec 15. J Clin Pharmacol. 2012. PMID: 22174434 Free PMC article. Clinical Trial.
-
CYP2D6 and tamoxifen: DNA matters in breast cancer.Nat Rev Cancer. 2009 Aug;9(8):576-86. doi: 10.1038/nrc2683. Nat Rev Cancer. 2009. PMID: 19629072 Review.
References
-
- Anderson GL, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291:1701–1712. - PubMed
-
- Herrington DM, et al. Estrogen-receptor polymorphisms and effects of estrogen replacement on high-density lipoprotein cholesterol in women with coronary disease. N. Engl. J. Med. 2002;346:967–974. - PubMed
-
- Furr BJ, Jordan VC. The pharmacology and clinical uses of tamoxifen. Pharmacol. Ther. 1984;25:127–205. - PubMed
-
- Costantino JP, Kuller LH, Ives DG, Fisher B, Dignam J. Coronary heart disease mortality and adjuvant tamoxifen therapy. J. Natl. Cancer Inst. 1997;89:776–782. - PubMed
-
- Center for Drug Evaluation and Research. < http://www.FDA.GOV/CDER/NEWS/TAMOXIFEN/DEFAULT.htm>.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

