Antiretroviral treatment and prevention of peripartum and postnatal HIV transmission in West Africa: evaluation of a two-tiered approach
- PMID: 17713983
- PMCID: PMC1949842
- DOI: 10.1371/journal.pmed.0040257
Antiretroviral treatment and prevention of peripartum and postnatal HIV transmission in West Africa: evaluation of a two-tiered approach
Abstract
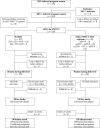
Background: Highly active antiretroviral treatment (HAART) has only been recently recommended for HIV-infected pregnant women requiring treatment for their own health in resource-limited settings. However, there are few documented experiences from African countries. We evaluated the short-term (4 wk) and long-term (12 mo) effectiveness of a two-tiered strategy of prevention of mother-to-child transmission of HIV (PMTCT) in Africa: women meeting the eligibility criteria of the World Health Organization (WHO) received HAART, and women with less advanced HIV disease received short-course antiretroviral (scARV) PMTCT regimens.
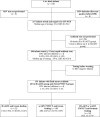
Methods and findings: The MTCT-Plus Initiative is a multi-country, family-centred HIV care and treatment program for pregnant and postpartum women and their families. Pregnant women enrolled in Abidjan, Côte d'Ivoire received either HAART for their own health or short-course antiretroviral (scARV) PMTCT regimens according to their clinical and immunological status. Plasma HIV-RNA viral load (VL) was measured to diagnose peripartum infection when infants were 4 wk of age, and HIV final status was documented either by rapid antibody testing when infants were aged > or = 12 mo or by plasma VL earlier. The Kaplan-Meier method was used to estimate the rate of HIV transmission and HIV-free survival. Between August 2003 and June 2005, 107 women began HAART at a median of 30 wk of gestation, 102 of them with zidovudine (ZDV), lamivudine (3TC), and nevirapine (NVP) and they continued treatment postpartum; 143 other women received scARV for PMTCT, 103 of them with sc(ZDV+3TC) with single-dose NVP during labour. Most (75%) of the infants were breast-fed for a median of 5 mo. Overall, the rate of peripartum HIV transmission was 2.2% (95% confidence interval [CI] 0.3%-4.2%) and the cumulative rate at 12 mo was 5.7% (95% CI 2.5%-9.0%). The overall probability of infant death or infection with HIV was 4.3% (95% CI 1.7%-7.0%) at age week 4 wk and 11.7% (95% CI 7.5%-15.9%) at 12 mo.
Conclusions: This two-tiered strategy appears to be safe and highly effective for short- and long-term PMTCT in resource-constrained settings. These results indicate a further benefit of access to HAART for pregnant women who need treatment for their own health.
Conflict of interest statement
Figures
Similar articles
-
Maternal 12-month response to antiretroviral therapy following prevention of mother-to-child transmission of HIV type 1, Ivory Coast, 2003-2006.Clin Infect Dis. 2008 Feb 15;46(4):611-21. doi: 10.1086/526780. Clin Infect Dis. 2008. PMID: 18197758
-
Antiretroviral therapy in pregnant women with advanced HIV disease and pregnancy outcomes in Abidjan, Côte d'Ivoire.AIDS. 2008 Sep 12;22(14):1815-20. doi: 10.1097/QAD.0b013e32830b8ab9. AIDS. 2008. PMID: 18753864
-
Treatment acceleration program and the experience of the DREAM program in prevention of mother-to-child transmission of HIV.AIDS. 2007 Jul;21 Suppl 4:S65-71. doi: 10.1097/01.aids.0000279708.09180.f5. AIDS. 2007. PMID: 17620755
-
International recommendations on antiretroviral drugs for treatment of HIV-infected women and prevention of mother-to-child HIV transmission in resource-limited settings: 2006 update.Am J Obstet Gynecol. 2007 Sep;197(3 Suppl):S42-55. doi: 10.1016/j.ajog.2007.03.001. Am J Obstet Gynecol. 2007. PMID: 17825650 Review.
-
Preventing mother-to-child transmission of HIV: successes and challenges.BJOG. 2005 Sep;112(9):1196-203. doi: 10.1111/j.1471-0528.2005.00716.x. BJOG. 2005. PMID: 16101596 Review.
Cited by
-
Cost-effectiveness of World Health Organization 2010 guidelines for prevention of mother-to-child HIV transmission in Zimbabwe.Clin Infect Dis. 2013 Feb;56(3):430-46. doi: 10.1093/cid/cis858. Epub 2012 Nov 30. Clin Infect Dis. 2013. PMID: 23204035 Free PMC article.
-
Factors associated with access to HIV care and treatment in a prevention of mother to child transmission programme in urban Zimbabwe.J Int AIDS Soc. 2010 Oct 6;13:38. doi: 10.1186/1758-2652-13-38. J Int AIDS Soc. 2010. PMID: 20925943 Free PMC article.
-
Antiretroviral Therapy Helps HIV-Positive Women Navigate Social Expectations for and Clinical Recommendations against Childbearing in Uganda.AIDS Res Treat. 2014;2014:626120. doi: 10.1155/2014/626120. Epub 2014 Sep 18. AIDS Res Treat. 2014. PMID: 25328693 Free PMC article.
-
HIV prevention is not enough: child survival in the context of prevention of mother to child HIV transmission.J Int AIDS Soc. 2009 Dec 11;12:36. doi: 10.1186/1758-2652-12-36. J Int AIDS Soc. 2009. PMID: 20015345 Free PMC article.
-
Highly active antiretroviral therapy versus zidovudine for prevention of mother-to-child transmission in a programmatic setting, Botswana.J Acquir Immune Defic Syndr. 2011 Nov 1;58(3):353-7. doi: 10.1097/QAI.0b013e31822d4063. J Acquir Immune Defic Syndr. 2011. PMID: 21792062 Free PMC article.
References
-
- UNAIDS. 2006 Report on the global AIDS epidemic. 2006. Available: http://www.unaids.org/en/HIV_data/2006GlobalReport/default.asp. Accessed 28 February 2007.
-
- European Collaborative Study Group. Mother-to-child transmission of HIV infection in the era of highly active antiretroviral therapy. Clin Infect Dis. 2005;40:458–465. - PubMed
-
- Cooper ER, Charurat M, Mofenson L, Hanson IC, Pitt J, et al. Combination antiretroviral strategies for the treatment of pregnant HIV-1-infected women and prevention of perinatal HIV-1 transmission. J Acquir Immune Defic Syndr. 2002;29:484–494. - PubMed
-
- Wiktor SZ, Ekpini E, Karon JM, Nkengasong J, Maurice C, et al. Short-course oral zidovudine for prevention of mother-to-child transmission of HIV-1 in Abidjan, Cote d'Ivoire: A randomised trial. Lancet. 1999;353:781–785. - PubMed
-
- Petra Study Team. Efficacy of three short-course regimens of zidovudine and lamivudine in preventing early and late transmission of HIV-1 from mother to child in Tanzania, South Africa, and Uganda (Petra study): A randomised, double-blind, placebo-controlled trial. Lancet. 2002;359:1178–1186. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

