Trafficking of siderophore transporters in Saccharomyces cerevisiae and intracellular fate of ferrioxamine B conjugates
- PMID: 17714436
- PMCID: PMC2171038
- DOI: 10.1111/j.1600-0854.2007.00627.x
Trafficking of siderophore transporters in Saccharomyces cerevisiae and intracellular fate of ferrioxamine B conjugates
Abstract
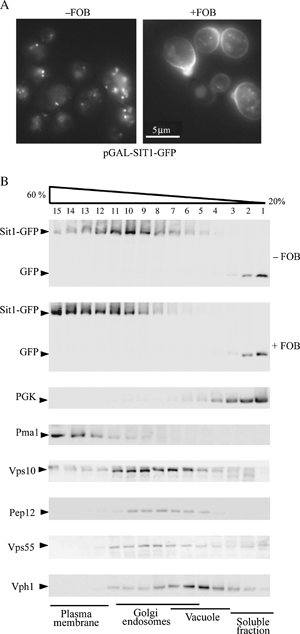
We have studied the intracellular trafficking of Sit1 [ferrioxamine B (FOB) transporter] and Enb1 (enterobactin transporter) in Saccharomyces cerevisiae using green fluorescent protein (GFP) fusion proteins. Enb1 was constitutively targeted to the plasma membrane. Sit1 was essentially targeted to the vacuolar degradation pathway when synthesized in the absence of substrate. Massive plasma membrane sorting of Sit1 was induced by various siderophore substrates of Sit1, and by coprogen, which is not a substrate of Sit1. Thus, different siderophore transporters use different regulated trafficking processes. We also studied the fate of Sit1-mediated internalized siderophores. Ferrioxamine B was recovered in isolated vacuolar fractions, where it could be detected spectrophotometrically. Ferrioxamine B coupled to an inhibitor of mitochondrial protoporphyrinogen oxidase (acifluorfen) could not reach its target unless the cells were disrupted, confirming the tight compartmentalization of siderophores within cells. Ferrioxamine B coupled to a fluorescent moiety, FOB-nitrobenz-2-oxa-1,3-diazole, used as a Sit1-dependent iron source, accumulated in the vacuolar lumen even in mutants displaying a steady-state accumulation of Sit1 at the plasma membrane or in endosomal compartments. Thus, the fates of siderophore transporters and siderophores diverge early in the trafficking process.
Figures



Similar articles
-
Substrate- and ubiquitin-dependent trafficking of the yeast siderophore transporter Sit1.Traffic. 2008 Aug;9(8):1372-91. doi: 10.1111/j.1600-0854.2008.00766.x. Epub 2008 May 17. Traffic. 2008. PMID: 18489705
-
Siderophore-mediated iron uptake in Saccharomyces cerevisiae: the SIT1 gene encodes a ferrioxamine B permease that belongs to the major facilitator superfamily.Microbiology (Reading). 1998 Dec;144 ( Pt 12):3455-3462. doi: 10.1099/00221287-144-12-3455. Microbiology (Reading). 1998. PMID: 9884238
-
Physical interaction between Sit1 and Aft1 upregulates FOB uptake activity by inhibiting protein degradation of Sit1 in Saccharomyces cerevisiae.FEMS Yeast Res. 2015 Nov;15(7):fov080. doi: 10.1093/femsyr/fov080. Epub 2015 Aug 30. FEMS Yeast Res. 2015. PMID: 26323600
-
The response to iron deprivation in Saccharomyces cerevisiae: expression of siderophore-based systems of iron uptake.Biochem Soc Trans. 2002 Aug;30(4):698-702. doi: 10.1042/bst0300698. Biochem Soc Trans. 2002. PMID: 12196168 Review.
-
Structure, function and binding selectivity and stereoselectivity of siderophore-iron outer membrane transporters.Curr Top Membr. 2012;69:37-66. doi: 10.1016/B978-0-12-394390-3.00002-1. Curr Top Membr. 2012. PMID: 23046646 Review.
Cited by
-
Ear1p and Ssh4p are new adaptors of the ubiquitin ligase Rsp5p for cargo ubiquitylation and sorting at multivesicular bodies.Mol Biol Cell. 2008 Jun;19(6):2379-88. doi: 10.1091/mbc.e08-01-0068. Epub 2008 Mar 26. Mol Biol Cell. 2008. PMID: 18367543 Free PMC article.
-
Response to iron deprivation in Saccharomyces cerevisiae.Eukaryot Cell. 2008 Jan;7(1):20-7. doi: 10.1128/EC.00354-07. Epub 2007 Nov 9. Eukaryot Cell. 2008. PMID: 17993568 Free PMC article. Review. No abstract available.
-
A dual role for K63-linked ubiquitin chains in multivesicular body biogenesis and cargo sorting.Mol Biol Cell. 2012 Jun;23(11):2170-83. doi: 10.1091/mbc.E11-10-0891. Epub 2012 Apr 4. Mol Biol Cell. 2012. PMID: 22493318 Free PMC article.
-
Identification and characterization of a novel-type ferric siderophore reductase from a gram-positive extremophile.J Biol Chem. 2011 Jan 21;286(3):2245-60. doi: 10.1074/jbc.M110.192468. Epub 2010 Nov 4. J Biol Chem. 2011. PMID: 21051545 Free PMC article.
-
Alterations of protein expression in conditions of copper-deprivation for Paracoccidioides lutzii in the presence of extracellular matrix components.BMC Microbiol. 2014 Dec 13;14:302. doi: 10.1186/s12866-014-0302-7. BMC Microbiol. 2014. PMID: 25609357 Free PMC article.
References
-
- Lesuisse E, Labbe P. Reductive and non-reductive mechanisms of iron assimilation by the yeast Saccharomyces cerevisiae. J Gen Microbiol. 1989;135:257–263. - PubMed
-
- De Luca NG, Wood PM. Iron uptake by fungi: contrasted mechanisms with internal or external reduction. Adv Microb Physiol. 2000;43:39–74. - PubMed
-
- Yun CW, Ferea T, Rashford J, Ardon O, Brown PO, Botstein D, Kaplan J, Philpott CC. Desferrioxamine-mediated iron uptake in Saccharomyces cerevisiae. Evidence for two pathways of iron uptake. J Biol Chem. 2000;275:10709–10715. - PubMed
-
- Kosman DJ. Molecular mechanisms of iron uptake in fungi. Mol Microbiol. 2003;47:1185–1197. - PubMed
-
- Lesuisse E, Raguzzi F, Crichton RR. Iron uptake by the yeast Saccharomyces cerevisiae: involvement of a reduction step. J Gen Microbiol. 1987;133:3229–3236. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

