Slit modulates cerebrovascular inflammation and mediates neuroprotection against global cerebral ischemia
- PMID: 17714707
- PMCID: PMC2227314
- DOI: 10.1016/j.expneurol.2007.06.028
Slit modulates cerebrovascular inflammation and mediates neuroprotection against global cerebral ischemia
Abstract
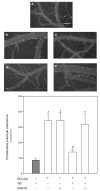
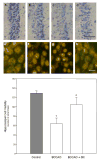
Cerebrovascular inflammation contributes to secondary brain injury following ischemia. Recent in vitro studies of cell migration and molecular guidance mechanisms have indicated that the Slit family of secreted proteins can exert repellant effects on leukocyte recruitment in response to chemoattractants. Utilizing intravital microscopy, we addressed the role of Slit in modulating leukocyte dynamics in the mouse cortical venular microcirculation in vivo following TNFalpha application or global cerebral ischemia. We also studied whether Slit affected neuronal survival in the mouse global ischemia model as well as in mixed neuronal-glial cultures subjected to oxygen-glucose deprivation. We found that systemically administered Slit significantly attenuated cerebral microvessel leukocyte-endothelial adherence occurring 4 h after TNFalpha and 24 h after global cerebral ischemia. Administration of RoboN, the soluble receptor for Slit, exacerbated the acute chemotactic response to TNFalpha. These findings are indicative of a tonic repellant effect of endogenous Slit in brain under acute proinflammatory conditions. Three days of continuous systemic administration of Slit following global ischemia significantly attenuated the delayed neuronal death of hippocampal CA1 pyramidal cells. Moreover, Slit abrogated neuronal death in mixed neuronal-glial cultures exposed to oxygen-glucose deprivation. The ability of Slit to reduce the recruitment of immune cells to ischemic brain and to provide cytoprotective effects suggests that this protein may serve as a novel anti-inflammatory and neuroprotective target for stroke therapy.
Figures


References
-
- Adamson P, Etienne S, Couraud PO, Calder V, Greenwood J. Lymphocyte migration through brain endothelial cell monolayers involves signaling through endothelial ICAM-1 via a rho-dependent pathway. J Immunol. 1999;162:2964–2973. - PubMed
-
- Akopov SE, Simonian NA, Grigorian GS. Dynamics of polymorphonuclear leukocyte accumulation in acute cerebral infarction and their correlation with brain tissue damage. Stroke. 1996;27:1739–1743. - PubMed
-
- Alon R, Grabovsky V, Feigelson S. Chemokine induction of integrin adhesiveness on rolling and arrested leukocytes local signaling events or global stepwise activation? Microcirculation. 2003;10:297–311. - PubMed
-
- Altay T, Gonzales ER, Park TS, Gidday JM. Cerebrovascular inflammation after brief episodic hypoxia: modulation by neuronal and endothelial nitric oxide synthase. J Appl Physiol. 2004;96:1223–1230. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

