Iron metabolism at the host pathogen interface: lipocalin 2 and the pathogen-associated iroA gene cluster
- PMID: 17714976
- PMCID: PMC2695446
- DOI: 10.1016/j.biocel.2007.07.003
Iron metabolism at the host pathogen interface: lipocalin 2 and the pathogen-associated iroA gene cluster
Abstract
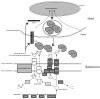
The host innate immune defense protein lipocalin 2 binds bacterial enterobactin siderophores to limit bacterial iron acquisition. To counteract this host defense mechanism bacteria have acquired the iroA gene cluster, which encodes enzymatic machinery and transporters that revitalize enterobactin in the form of salmochelin. The iroB enzyme introduces glucosyl residues at the C5 site on 2,3-dihydroxybenzoylserine moieties of enterobactin and thereby prevents lipocalin 2 binding. Additional strategies to evade lipocalin 2 have evolved in other bacteria, such as Mycobacteria tuberculosis and Bacillus anthracis. Targeting these specialized bacterial evasion strategy may provide a mechanism to reinvigorate lipocalin 2 in defense against specific pathogens.
Figures
References
-
- Baumler AJ, Tsolis RM, van der Velden AW, Stojiljkovic I, Anic S, Heffron F. Identification of a new iron regulated locus of Salmonella typhi. Gene. 1996;183(1–2):207–213. - PubMed
-
- Bister B, Bischoff D, Nicholson GJ, Valdebenito M, Schneider K, Winkelmann G, et al. The structure of salmochelins: C-glucosylated enterobactins of Salmonella enterica. Biometals. 2004;17(4):471–481. - PubMed
-
- Devireddy LR, Gazin C, Zhu X, Green MR. A cell-surface receptor for lipocalin 24p3 selectively mediates apoptosis and iron uptake. Cell. 2005;123(7):1293–1305. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

