The capsid and tegument of the alphaherpesviruses are linked by an interaction between the UL25 and VP1/2 proteins
- PMID: 17715218
- PMCID: PMC2168758
- DOI: 10.1128/JVI.01113-07
The capsid and tegument of the alphaherpesviruses are linked by an interaction between the UL25 and VP1/2 proteins
Abstract
How alphaherpesvirus capsids acquire tegument proteins remains a key question in viral assembly. Using pseudorabies virus (PRV), we have previously shown that the 62 carboxy-terminal amino acids of the VP1/2 large tegument protein are essential for viral propagation and when transiently expressed as a fusion to green fluorescent protein relocalize to nuclear capsid assemblons following viral infection. Here, we show that localization of the VP1/2 capsid-binding domain (VP1/2cbd) into assemblons is conserved in herpes simplex virus type 1 (HSV-1) and that this recruitment is specifically on capsids. Using a mutant virus screen, we find that the protein product of the UL25 gene is essential for VP1/2cbd association with capsids. An interaction between UL25 and VP1/2 was corroborated by coimmunoprecipitation from cells transiently expressing either HSV-1 or PRV proteins. Taken together, these findings suggest that the essential function of the VP1/2 carboxy terminus is to anchor the VP1/2 tegument protein to capsids. Furthermore, UL25 encodes a multifunctional capsid protein involved in not only encapsidation, as previously described, but also tegumentation.
Figures
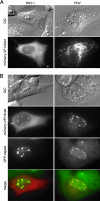
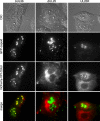


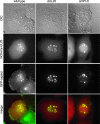
Similar articles
-
The capsid-associated UL25 protein of the alphaherpesvirus pseudorabies virus is nonessential for cleavage and encapsidation of genomic DNA but is required for nuclear egress of capsids.J Virol. 2006 Jul;80(13):6235-46. doi: 10.1128/JVI.02662-05. J Virol. 2006. PMID: 16775311 Free PMC article.
-
Role of the UL25 protein in herpes simplex virus DNA encapsidation.J Virol. 2009 Jan;83(1):47-57. doi: 10.1128/JVI.01889-08. Epub 2008 Oct 22. J Virol. 2009. PMID: 18945788 Free PMC article.
-
Identification of an essential domain in the herpesvirus VP1/2 tegument protein: the carboxy terminus directs incorporation into capsid assemblons.J Virol. 2006 Dec;80(24):12086-94. doi: 10.1128/JVI.01184-06. Epub 2006 Sep 27. J Virol. 2006. PMID: 17005660 Free PMC article.
-
Tegument Assembly and Secondary Envelopment of Alphaherpesviruses.Viruses. 2015 Sep 18;7(9):5084-114. doi: 10.3390/v7092861. Viruses. 2015. PMID: 26393641 Free PMC article. Review.
-
A Hitchhiker's Guide Through the Cell: The World According to the Capsids of Alphaherpesviruses.Annu Rev Virol. 2024 Sep;11(1):215-238. doi: 10.1146/annurev-virology-100422-022751. Epub 2024 Aug 30. Annu Rev Virol. 2024. PMID: 38954634 Review.
Cited by
-
The herpesvirus VP1/2 protein is an effector of dynein-mediated capsid transport and neuroinvasion.Cell Host Microbe. 2013 Feb 13;13(2):193-203. doi: 10.1016/j.chom.2013.01.009. Cell Host Microbe. 2013. PMID: 23414759 Free PMC article.
-
The Role of VP16 in the Life Cycle of Alphaherpesviruses.Front Microbiol. 2020 Aug 18;11:1910. doi: 10.3389/fmicb.2020.01910. eCollection 2020. Front Microbiol. 2020. PMID: 33013729 Free PMC article. Review.
-
Experimental Dissection of the Lytic Replication Cycles of Herpes Simplex Viruses in vitro.Front Microbiol. 2018 Oct 11;9:2406. doi: 10.3389/fmicb.2018.02406. eCollection 2018. Front Microbiol. 2018. PMID: 30386309 Free PMC article. Review.
-
Comprehensive Analysis of the Tegument Proteins Involved in Capsid Transport and Virion Morphogenesis of Alpha, Beta and Gamma Herpesviruses.Viruses. 2023 Oct 6;15(10):2058. doi: 10.3390/v15102058. Viruses. 2023. PMID: 37896835 Free PMC article. Review.
-
Crystal structure of the herpesvirus inner tegument protein UL37 supports its essential role in control of viral trafficking.J Virol. 2014 May;88(10):5462-73. doi: 10.1128/JVI.00163-14. Epub 2014 Mar 5. J Virol. 2014. PMID: 24599989 Free PMC article.
References
-
- Abramoff, M. D., P. J. Magelhaes, and S. J. Ram. 2004. Image processing with Image J. Biophotonics Int. 11:36-42.
-
- Addison, C., F. J. Rixon, J. W. Palfreyman, M. O'Hara, and V. G. Preston. 1984. Characterisation of a herpes simplex virus type 1 mutant which has a temperature-sensitive defect in penetration of cells and assembly of capsids. Virology 138:246-259. - PubMed
-
- Ali, M. A., B. Forghani, and E. M. Cantin. 1996. Characterization of an essential HSV-1 protein encoded by the UL25 gene reported to be involved in virus penetration and capsid assembly. Virology 216:278-283. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

