Neuroinvasion of fluorescein-positive monocytes in acute simian immunodeficiency virus infection
- PMID: 17715237
- PMCID: PMC2168770
- DOI: 10.1128/JVI.00133-07
Neuroinvasion of fluorescein-positive monocytes in acute simian immunodeficiency virus infection
Abstract
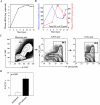
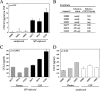
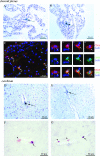
Monocytes and macrophages play a central role in the pathogenesis of human immunodeficiency virus (HIV)-associated dementia. They represent prominent targets for HIV infection and are thought to facilitate viral neuroinvasion and neuroinflammatory processes. However, many aspects regarding monocyte brain recruitment in HIV infection remain undefined. The nonhuman primate model of AIDS is uniquely suited for examination of the role of monocytes in the pathogenesis of AIDS-associated encephalitis. Nevertheless, an approach to monitor cell migration from peripheral blood into the central nervous system (CNS) in primates had been lacking. Here, upon autologous transfer of fluorescein dye-labeled leukocytes, we demonstrate the trafficking of dye-positive monocytes into the choroid plexus stromata and perivascular spaces in the cerebra of rhesus macaques acutely infected with simian immunodeficiency virus between days 12 and 14 postinfection (p.i.). Dye-positive cells that had migrated expressed the monocyte activation marker CD16 and the macrophage marker CD68. Monocyte neuroinvasion coincided with the presence of the virus in brain tissue and cerebrospinal fluid and with the induction of the proinflammatory mediators CXCL9/MIG and CCL2/MCP-1 in the CNS. Prior to neuroinfiltration, plasma viral load levels peaked on day 11 p.i. Furthermore, the numbers of peripheral blood monocytes rapidly increased between days 4 and 8 p.i., and circulating monocytes exhibited increased functional capacity to produce CCL2/MCP-1. Our findings demonstrate acute monocyte brain infiltration in an animal model of AIDS. Such studies facilitate future examinations of the migratory profile of CNS-homing monocytes, the role of monocytes in virus import into the brain, and the disruption of blood-cerebrospinal fluid and blood-brain barrier functions in primates.
Figures
Similar articles
-
Monocyte/macrophage trafficking in acquired immunodeficiency syndrome encephalitis: lessons from human and nonhuman primate studies.J Neurovirol. 2008 Aug;14(4):318-26. doi: 10.1080/13550280802132857. J Neurovirol. 2008. PMID: 18780233 Free PMC article. Review.
-
Neuroinvasion by simian immunodeficiency virus coincides with increased numbers of perivascular macrophages/microglia and intrathecal immune activation.J Neurovirol. 1996 Dec;2(6):423-32. doi: 10.3109/13550289609146909. J Neurovirol. 1996. PMID: 8972425
-
Neuroprotective and anti-human immunodeficiency virus activity of minocycline.JAMA. 2005 Apr 27;293(16):2003-11. doi: 10.1001/jama.293.16.2003. JAMA. 2005. PMID: 15855434
-
Dynamics of simian immunodeficiency virus populations in blood and cerebrospinal fluid over the full course of infection.J Infect Dis. 2007 Oct 1;196(7):1058-67. doi: 10.1086/520819. Epub 2007 Aug 29. J Infect Dis. 2007. PMID: 17763329
-
Searching for clues: tracking the pathogenesis of human immunodeficiency virus central nervous system disease by use of an accelerated, consistent simian immunodeficiency virus macaque model.J Infect Dis. 2002 Dec 1;186 Suppl 2:S199-208. doi: 10.1086/344938. J Infect Dis. 2002. PMID: 12424698 Review.
Cited by
-
Molecular mechanisms of neuroinvasion by monocytes-macrophages in HIV-1 infection.Retrovirology. 2010 Apr 7;7:30. doi: 10.1186/1742-4690-7-30. Retrovirology. 2010. PMID: 20374632 Free PMC article. Review.
-
Coordinated regulation of SIV replication and immune responses in the CNS.PLoS One. 2009 Dec 17;4(12):e8129. doi: 10.1371/journal.pone.0008129. PLoS One. 2009. PMID: 20019816 Free PMC article.
-
Alterations in brain metabolism during the first year of HIV infection.J Neurovirol. 2011 Jun;17(3):220-9. doi: 10.1007/s13365-011-0030-9. Epub 2011 Apr 15. J Neurovirol. 2011. PMID: 21494901 Free PMC article.
-
Recently infiltrating MAC387(+) monocytes/macrophages a third macrophage population involved in SIV and HIV encephalitic lesion formation.Am J Pathol. 2011 May;178(5):2121-35. doi: 10.1016/j.ajpath.2011.01.023. Am J Pathol. 2011. PMID: 21514427 Free PMC article.
-
Virus Multiplicity of Infection Affects Type I Interferon Subtype Induction Profiles and Interferon-Stimulated Genes.J Virol. 2015 Nov;89(22):11534-48. doi: 10.1128/JVI.01727-15. Epub 2015 Sep 9. J Virol. 2015. PMID: 26355085 Free PMC article.
References
-
- Ancuta, P., P. Autissier, A. Wurcel, T. Zaman, D. Stone, and D. Gabuzda. 2006. CD16+ monocyte-derived macrophages activate resting T cells for HIV infection by producing CCR3 and CCR4 ligands. J. Immunol. 176:5760-5771. - PubMed
-
- Ancuta, P., J. Wang, and D. Gabuzda. 2006. CD16+ monocytes produce IL-6, CCL2, and matrix metalloproteinase-9 upon interaction with CX3CL1-expressing endothelial cells. J. Leukoc. Biol. 80:1156-1164. - PubMed
-
- Asensio, V. C., J. Maier, R. Milner, K. Boztug, C. Kincaid, M. Moulard, C. Phillipson, K. Lindsley, T. Krucker, H. S. Fox, and I. L. Campbell. 2001. Interferon-independent, human immunodeficiency virus type 1 gp120-mediated induction of CXCL10/IP-10 gene expression by astrocytes in vivo and in vitro. J. Virol. 75:7067-7077. - PMC - PubMed
-
- Baumgarth, N., R. Szubin, G. M. Dolganov, M. R. Watnik, D. Greenspan, M. Da Costa, J. M. Palefsky, R. Jordan, M. Roederer, and J. S. Greenspan. 2004. Highly tissue substructure-specific effects of human papilloma virus in mucosa of HIV-infected patients revealed by laser-dissection microscopy-assisted gene expression profiling. Am. J. Pathol. 165:707-718. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

