Three-dimensional structure of the human cytomegalovirus cytoplasmic virion assembly complex includes a reoriented secretory apparatus
- PMID: 17715239
- PMCID: PMC2168812
- DOI: 10.1128/JVI.01077-07
Three-dimensional structure of the human cytomegalovirus cytoplasmic virion assembly complex includes a reoriented secretory apparatus
Abstract
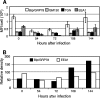
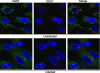
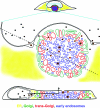
Human cytomegalovirus (HCMV) induces profound changes in infected cell morphology, including a large cytoplasmic inclusion that corresponds to the virion assembly complex (AC). In electron micrographs, the AC is a highly vacuolated part of the cytoplasm. Markers of cellular secretory organelles have been visualized at the outer edge of the AC, and we recently showed that a marker for early endosomes (i.e., early endosome antigen 1) localizes to the center of the AC. Here, we examined the relationship between the AC and components of the secretory apparatus, studied temporal aspects of the dramatic infection-induced cytoplasmic remodeling, examined the three-dimensional structure of the AC, and considered the implications of our observations for models of HCMV virion maturation and egress. We made three major observations. First, in addition to being relocated, the expression levels of some organelle markers change markedly during the period while the AC is developing. Second, based on three-dimensional reconstructions from z-series confocal microscopic images, the observed concentric rings of vesicles derived from the several compartments (Golgi bodies, the trans-Golgi network [TGN], and early endosomes) are arranged as nested cylinders of organelle-specific vesicles. Third, the membrane protein biosynthetic and exocytic pathways from the endoplasmic reticulum to the Golgi bodies, TGN, and early endosomes are in an unusual arrangement that nonetheless allows for a conventional order of biosynthesis and transport. Our model of AC structure suggests a mechanism by which the virus can regulate the order of tegument assembly.
Figures



References
-
- Azzeh, M., A. Honigman, A. Taraboulos, A. Rouvinski, and D. G. Wolf. 2006. Structural changes in human cytomegalovirus cytoplasmic assembly sites in the absence of UL97 kinase activity. Virology 354:69-79. - PubMed
-
- Barr, F. A. 1999. A novel Rab6-interacting domain defines a family of Golgi-targeted coiled-coil proteins. Curr. Biol. 9:381-384. - PubMed
-
- Barr, F. A., and B. Short. 2003. Golgins in the structure and dynamics of the Golgi apparatus. Curr. Opin. Cell Biol. 15:405-413. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

