An 11-amino acid beta-hairpin loop in the cytoplasmic domain of band 3 is responsible for ankyrin binding in mouse erythrocytes
- PMID: 17715300
- PMCID: PMC1950715
- DOI: 10.1073/pnas.0706266104
An 11-amino acid beta-hairpin loop in the cytoplasmic domain of band 3 is responsible for ankyrin binding in mouse erythrocytes
Abstract
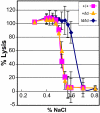
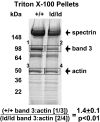
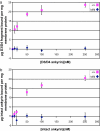
The best-studied cytoskeletal system is the inner surface of the erythrocyte membrane, which provides an erythrocyte with the structural support needed to be stable yet flexible as it passes through the circulation. Current structural models predict that the spectrin-actin-based cytoskeletal network is attached to the plasma membrane through interactions of the protein ankyrin, which binds to both spectrin and the cytoplasmic domain of the transmembrane protein band 3. The crystal structure of the cytoplasmic domain of band 3 predicted that the ankyrin binding site was located on a beta-hairpin loop in the cytoplasmic domain. In vitro, deletion of this loop eliminated ankyrin affinity for band 3 without affecting any other protein-band 3 interaction. To evaluate the importance of the ankyrin-band 3 linkage to membrane properties in vivo, we generated mice with the nucleotides encoding the 11-aa beta-hairpin loop in the mouse Slc4a1 gene replaced with sequence encoding a diglycine bridge. Mice homozygous for the loop deletion were viable with mildly spherocytic and osmotically fragile erythrocytes. In vitro, homozygous ld/ld erythrocytes were incapable of binding ankyrin, but contrary to all previous predictions, abolishing the ankyrin-band 3 linkage destabilized the erythrocyte membrane to a lesser degree than complete deficiencies of either band 3 or ankyrin. Our data indicate that as yet uncharacterized interactions between other membrane proteins must significantly contribute to linkage of the spectrin-actin-based membrane cytoskeleton to the plasma membrane.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


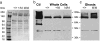
Similar articles
-
Identification of a critical ankyrin-binding loop on the cytoplasmic domain of erythrocyte membrane band 3 by crystal structure analysis and site-directed mutagenesis.J Biol Chem. 2003 Feb 28;278(9):6879-84. doi: 10.1074/jbc.M211137200. Epub 2002 Dec 12. J Biol Chem. 2003. PMID: 12482869
-
Identification of contact sites between ankyrin and band 3 in the human erythrocyte membrane.Biochemistry. 2012 Aug 28;51(34):6838-46. doi: 10.1021/bi300693k. Epub 2012 Aug 14. Biochemistry. 2012. PMID: 22861190 Free PMC article.
-
Determination of structural models of the complex between the cytoplasmic domain of erythrocyte band 3 and ankyrin-R repeats 13-24.J Biol Chem. 2011 Jun 10;286(23):20746-57. doi: 10.1074/jbc.M111.230326. Epub 2011 Apr 14. J Biol Chem. 2011. PMID: 21493712 Free PMC article.
-
The molecular basis for membrane - cytoskeleton association in human erythrocytes.J Cell Biochem. 1982;18(1):49-65. doi: 10.1002/jcb.1982.240180106. J Cell Biochem. 1982. PMID: 6461664 Review.
-
[Molecular interactions of membrane proteins and erythrocyte deformability].Pathol Biol (Paris). 1984 Jun;32(6):717-35. Pathol Biol (Paris). 1984. PMID: 6235477 Review. French.
Cited by
-
Utility of multi-gene loci for forensic species diagnosis of blowflies.J Insect Sci. 2011;11:59. doi: 10.1673/031.011.5901. J Insect Sci. 2011. PMID: 21864153 Free PMC article.
-
Effect of radiographic contrast media on the spectrin/band3-network of the membrane skeleton of erythrocytes.PLoS One. 2014 Feb 24;9(2):e89512. doi: 10.1371/journal.pone.0089512. eCollection 2014. PLoS One. 2014. PMID: 24586837 Free PMC article.
-
Regulation of membrane-cytoskeletal interactions by tyrosine phosphorylation of erythrocyte band 3.Blood. 2011 Jun 2;117(22):5998-6006. doi: 10.1182/blood-2010-11-317024. Epub 2011 Apr 7. Blood. 2011. PMID: 21474668 Free PMC article.
-
Membrane domains based on ankyrin and spectrin associated with cell-cell interactions.Cold Spring Harb Perspect Biol. 2009 Dec;1(6):a003012. doi: 10.1101/cshperspect.a003012. Epub 2009 Aug 19. Cold Spring Harb Perspect Biol. 2009. PMID: 20457566 Free PMC article. Review.
-
Melatonin Protects Band 3 Protein in Human Erythrocytes against H2O2-Induced Oxidative Stress.Molecules. 2019 Jul 28;24(15):2741. doi: 10.3390/molecules24152741. Molecules. 2019. PMID: 31357737 Free PMC article.
References
-
- Lux SE. Nature. 1979;281:426–429. - PubMed
-
- Van Dort HM, Knowles DW, Chasis JA, Lee G, Mohandas N, Low PS. J Biol Chem. 2001;276:46968–46974. - PubMed
-
- Nicolas V, Le Van Kim C, Gane P, Birkenmeier C, Cartron JP, Colin Y, Mouro-Chanteloup I. J Biol Chem. 2003;278:25526–25533. - PubMed
-
- Nelson WJ, Veshnock PJ. Nature. 1987;328:533–536. - PubMed
-
- Li ZP, Burke EP, Frank JS, Bennett V, Philipson KD. J Biol Chem. 1993;268:11489–11491. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

