Immunotherapy targeting pathological tau conformers in a tangle mouse model reduces brain pathology with associated functional improvements
- PMID: 17715348
- PMCID: PMC6672191
- DOI: 10.1523/JNEUROSCI.2361-07.2007
Immunotherapy targeting pathological tau conformers in a tangle mouse model reduces brain pathology with associated functional improvements
Abstract
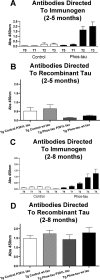
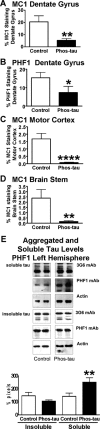
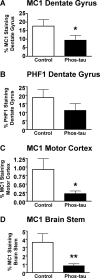
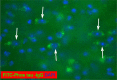
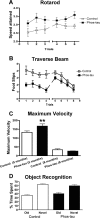
Immunotherapies for various neurodegenerative diseases have recently emerged as a promising approach for clearing pathological protein conformers in these disorders. This type of treatment has not been assessed in models that develop neuronal tau aggregates as observed in frontotemporal dementia and Alzheimer's disease. Here, we present that active immunization with a phosphorylated tau epitope, in P301L tangle model mice, reduces aggregated tau in the brain and slows progression of the tangle-related behavioral phenotype. Females had more tau pathology than males but were also more receptive to the immunotherapy. The tau antibodies generated in these animals recognized pathological tau on brain sections. Performance on behavioral assays that require extensive motor coordination correlated with tau pathology in corresponding brain areas, and antibody levels against the immunogen correlated inversely with tau pathology. Interestingly, age-dependent autoantibodies that recognized recombinant tau protein but not the immunogen were detected in the P301L mice. To confirm that anti-tau antibodies could enter the brain and bind to pathological tau, FITC-tagged antibodies purified from a P301L mouse, with a high antibody titer against the immunogen, were injected into the carotid artery of P301L mice. These antibodies were subsequently detected within the brain and colocalized with PHF1 and MC1 antibodies that recognize pathological tau. Currently, no treatment is available for clearing tau aggregates. Our present findings may lead to a novel therapy targeting one of the major hallmarks of Alzheimer's disease and frontotemporal dementia.
Figures





References
-
- Aihara N, Tanno H, Hall JJ, Pitts LH, Noble LJ. Immunocytochemical localization of immunoglobulins in the rat brain: relationship to the blood-brain barrier. J Comp Neurol. 1994;342:481–496. - PubMed
-
- Andoh T, Kuraishi Y. Direct action of immunoglobulin G on primary sensory neurons through Fc gamma receptor I. FASEB J. 2004;18:182–184. - PubMed
-
- Asuni AA, Knudsen E, Frangione B, Wisniewski T, Sigurdsson EM. Antibody mediated modulation of Aβ induced neurotoxicity in cell culture. Neurobiol Aging. 2004;25:S581–S582.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
