Class I histone deacetylase Thd1p promotes global chromatin condensation in Tetrahymena thermophila
- PMID: 17715364
- PMCID: PMC2043386
- DOI: 10.1128/EC.00217-07
Class I histone deacetylase Thd1p promotes global chromatin condensation in Tetrahymena thermophila
Abstract
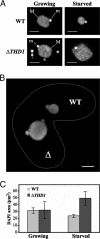
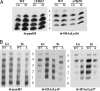
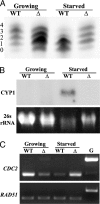
Class I histone deacetylases (HDACs) regulate DNA-templated processes such as transcription. They act both at specific loci and more generally across global chromatin, contributing to acetylation patterns that may underlie large-scale chromatin dynamics. Although hypoacetylation is correlated with highly condensed chromatin, little is known about the contribution of individual HDACs to chromatin condensation mechanisms. Using the ciliated protozoan Tetrahymena thermophila, we investigated the role of a specific class I HDAC, Tauhd1p, in the reversible condensation of global chromatin. In this system, the normal physiological response to cell starvation includes the widespread condensation of the macronuclear chromatin and general repression of gene transcription. We show that the chromatin in Thd1p-deficient cells failed to condense during starvation. The condensation failure correlated with aberrant hyperphosphorylation of histone H1 and the overexpression of CDC2, encoding the major histone H1 kinase. Changes in the rate of acetate turnover on core histones and in the distribution of acetylated lysines 9 and 23/27 on histone H3 isoforms that were found to correlate with normal chromatin condensation were absent from Thd1p mutant cells. These results point to a role for a class I HDAC in the formation of reversible higher-order chromatin structures and global genome compaction through mechanisms involving the regulation of H1 phosphorylation and core histone acetylation/deacetylation kinetics.
Figures



Similar articles
-
A class II histone deacetylase acts on newly synthesized histones in Tetrahymena.Eukaryot Cell. 2008 Mar;7(3):471-82. doi: 10.1128/EC.00409-07. Epub 2008 Jan 4. Eukaryot Cell. 2008. PMID: 18178773 Free PMC article.
-
Class I histone deacetylase Thd1p affects nuclear integrity in Tetrahymena thermophila.Eukaryot Cell. 2005 May;4(5):981-90. doi: 10.1128/EC.4.5.981-990.2005. Eukaryot Cell. 2005. PMID: 15879532 Free PMC article.
-
Developmentally regulated rpd3p homolog specific to the transcriptionally active macronucleus of vegetative Tetrahymena thermophila.Mol Cell Biol. 2000 Nov;20(22):8319-28. doi: 10.1128/MCB.20.22.8319-8328.2000. Mol Cell Biol. 2000. PMID: 11046129 Free PMC article.
-
Chromatin modifications remodel cardiac gene expression.Cardiovasc Res. 2014 Jul 1;103(1):7-16. doi: 10.1093/cvr/cvu122. Epub 2014 May 8. Cardiovasc Res. 2014. PMID: 24812277 Review.
-
Epigenetic control of ovarian function: the emerging role of histone modifications.Mol Cell Endocrinol. 2005 Nov 24;243(1-2):12-8. doi: 10.1016/j.mce.2005.09.005. Epub 2005 Oct 10. Mol Cell Endocrinol. 2005. PMID: 16219412 Review.
Cited by
-
Deacetylation and methylation at histone H3 lysine 9 (H3K9) coordinate chromosome condensation during cell cycle progression.Mol Cells. 2011 Apr;31(4):343-9. doi: 10.1007/s10059-011-0044-4. Epub 2011 Feb 2. Mol Cells. 2011. PMID: 21359677 Free PMC article.
-
A class II histone deacetylase acts on newly synthesized histones in Tetrahymena.Eukaryot Cell. 2008 Mar;7(3):471-82. doi: 10.1128/EC.00409-07. Epub 2008 Jan 4. Eukaryot Cell. 2008. PMID: 18178773 Free PMC article.
-
Genetic analysis of common variants in the HDAC2 gene with schizophrenia susceptibility in Han Chinese.J Hum Genet. 2015 Sep;60(9):479-84. doi: 10.1038/jhg.2015.66. Epub 2015 Jun 11. J Hum Genet. 2015. PMID: 26063464
-
Bioinformatic and proteomic analysis of bulk histones reveals PTM crosstalk and chromatin features.J Proteome Res. 2014 Jul 3;13(7):3330-7. doi: 10.1021/pr5001829. Epub 2014 Jun 13. J Proteome Res. 2014. PMID: 24894457 Free PMC article.
-
Adipocyte Metabolic Pathways Regulated by Diet Control the Female Germline Stem Cell Lineage in Drosophila melanogaster.Genetics. 2017 Jun;206(2):953-971. doi: 10.1534/genetics.117.201921. Epub 2017 Apr 10. Genetics. 2017. PMID: 28396508 Free PMC article.
References
-
- Allis, C. D., and M. A. Gorovsky. 1981. Histone phosphorylation in macro- and micronuclei of Tetrahymena thermophila. Biochemistry 20:3828-3833. - PubMed
-
- Annunziato, A. T., L. L. Frado, R. L. Seale, and C. L. Woodcock. 1988. Treatment with sodium butyrate inhibits the complete condensation of interphase chromatin. Chromosoma 96:132-138. - PubMed
-
- Calzone, R. J., V. A. Stathopoulos, D. Grass, M. A. Gorovsky, and R. C. Angerer. 1983. Regulation of protein synthesis in Tetrahymena: RNA sequence sets of growing and starved cells. J. Biol. Chem. 258:6899-6905. - PubMed
-
- Chicoine, L. G., and C. D. Allis. 1986. Regulation of histone acetylation during macronuclear differentiation in Tetrahymena: evidence for control at the level of acetylation and deacetylation. Dev. Biol. 116:477-485. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

