Role of actin cytoskeletal dynamics in activation of the cyclic AMP pathway and HWP1 gene expression in Candida albicans
- PMID: 17715368
- PMCID: PMC2043390
- DOI: 10.1128/EC.00188-07
Role of actin cytoskeletal dynamics in activation of the cyclic AMP pathway and HWP1 gene expression in Candida albicans
Abstract
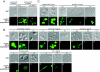
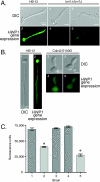
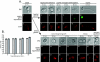
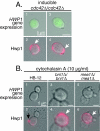
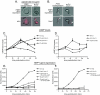
Changes in gene expression during reversible bud-hypha transitions of the opportunistic fungal pathogen Candida albicans permit adaptation to environmental conditions that are critical for proliferation in host tissues. Our previous work has shown that the hypha-specific adhesin gene HWP1 is up-regulated by the cyclic AMP (cAMP) signaling pathway. However, little is known about the potential influences of determinants of cell morphology on HWP1 gene expression. We found that blocking hypha formation with cytochalasin A, which destabilizes actin filaments, and with latrunculin A, which sequesters actin monomers, led to a loss of HWP1 gene expression. In contrast, high levels of HWP1 gene expression were observed when the F-actin stabilizer jasplakinolide was used to block hypha formation, suggesting that HWP1 expression could be regulated by actin structures. Mutants defective in formin-mediated nucleation of F-actin were reduced in HWP1 gene expression, providing genetic support for the importance of actin structures. Kinetic experiments with wild-type and actin-deficient cells revealed two distinct phases of HWP1 gene expression, with a slow, actin-independent phase preceding a fast, actin-dependent phase. Low levels of HWP1 gene expression that appeared to be independent of stabilized actin and cAMP signaling were detected using indirect immunofluorescence. A connection between actin structures and the cAMP signaling pathway was shown using hyper- and hypomorphic cAMP mutants, providing a possible mechanism for up-regulation of HWP1 gene expression by stabilized actin. The results reveal a new role for F-actin as a regulatory agent of hypha-specific gene expression at the bud-hypha transition.
Figures
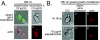



Similar articles
-
CAP1, an adenylate cyclase-associated protein gene, regulates bud-hypha transitions, filamentous growth, and cyclic AMP levels and is required for virulence of Candida albicans.J Bacteriol. 2001 May;183(10):3211-23. doi: 10.1128/JB.183.10.3211-3223.2001. J Bacteriol. 2001. PMID: 11325951 Free PMC article.
-
Gpr1, a putative G-protein-coupled receptor, regulates morphogenesis and hypha formation in the pathogenic fungus Candida albicans.Eukaryot Cell. 2004 Aug;3(4):919-31. doi: 10.1128/EC.3.4.919-931.2004. Eukaryot Cell. 2004. PMID: 15302825 Free PMC article.
-
A 368-base-pair cis-acting HWP1 promoter region, HCR, of Candida albicans confers hypha-specific gene regulation and binds architectural transcription factors Nhp6 and Gcf1p.Eukaryot Cell. 2007 Apr;6(4):693-709. doi: 10.1128/EC.00341-06. Epub 2007 Jan 12. Eukaryot Cell. 2007. PMID: 17220463 Free PMC article.
-
Adhesins in Candida albicans.Curr Opin Microbiol. 1999 Aug;2(4):353-7. doi: 10.1016/S1369-5274(99)80062-9. Curr Opin Microbiol. 1999. PMID: 10458989 Review.
-
Role of the actin cytoskeleton on epithelial Na+ channel regulation.Kidney Int. 1995 Oct;48(4):970-84. doi: 10.1038/ki.1995.379. Kidney Int. 1995. PMID: 8569107 Review.
Cited by
-
Signaling through Lrg1, Rho1 and Pkc1 Governs Candida albicans Morphogenesis in Response to Diverse Cues.PLoS Genet. 2016 Oct 27;12(10):e1006405. doi: 10.1371/journal.pgen.1006405. eCollection 2016 Oct. PLoS Genet. 2016. PMID: 27788136 Free PMC article.
-
The MARVEL domain protein Nce102 regulates actin organization and invasive growth of Candida albicans.mBio. 2013 Nov 26;4(6):e00723-13. doi: 10.1128/mBio.00723-13. mBio. 2013. PMID: 24281718 Free PMC article.
-
Filamentation Involves Two Overlapping, but Distinct, Programs of Filamentation in the Pathogenic Fungus Candida albicans.G3 (Bethesda). 2017 Nov 6;7(11):3797-3808. doi: 10.1534/g3.117.300224. G3 (Bethesda). 2017. PMID: 28951491 Free PMC article.
-
Whi2p links nutritional sensing to actin-dependent Ras-cAMP-PKA regulation and apoptosis in yeast.J Cell Sci. 2009 Mar 1;122(Pt 5):706-15. doi: 10.1242/jcs.042424. Epub 2009 Feb 10. J Cell Sci. 2009. PMID: 19208759 Free PMC article.
-
Rab11 and actin cytoskeleton participate in Giardia lamblia encystation, guiding the specific vesicles to the cyst wall.PLoS Negl Trop Dis. 2010 Jun 1;4(6):e697. doi: 10.1371/journal.pntd.0000697. PLoS Negl Trop Dis. 2010. PMID: 20532229 Free PMC article.
References
-
- Akashi, T., T. Kanbe, and K. Tanaka. 1994. The role of the cytoskeleton in the polarized growth of the germ tube in Candida albicans. Microbiology 140:271-280. - PubMed
-
- Anderson, J. M., and D. R. Soll. 1986. Differences in actin localization during bud and hypha formation in the yeast Candida albicans. J. Gen. Microbiol. 132:2035-2047. - PubMed
-
- Argimon, S., J. A. Wishart, R. Leng, S. Macaskill, A. Mavor, T. Alexandris, S. Nicholls, A. W. Knight, B. Enjalbert, R. Walmsley, F. C. Odds, N. A. Gow, and A. J. Brown. 2007. Developmental regulation of an adhesin gene during cellular morphogenesis in the fungal pathogen Candida albicans. Eukaryot. Cell 6:682-692. - PMC - PubMed
-
- Ayscough, K. R. 2000. Endocytosis and the development of cell polarity in yeast require a dynamic F-actin cytoskeleton. Curr. Biol. 10:1587-1590. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

