MLN3897, a novel CCR1 inhibitor, impairs osteoclastogenesis and inhibits the interaction of multiple myeloma cells and osteoclasts
- PMID: 17715391
- PMCID: PMC2077320
- DOI: 10.1182/blood-2007-05-093294
MLN3897, a novel CCR1 inhibitor, impairs osteoclastogenesis and inhibits the interaction of multiple myeloma cells and osteoclasts
Abstract
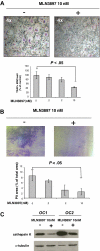
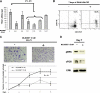
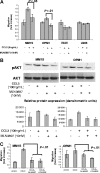
The interaction between osteoclasts (OCs) and multiple myeloma (MM) cells plays a key role in the pathogenesis of MM-related osteolytic bone disease (OBD). MM cells promote OC formation and, in turn, OCs enhance MM cell proliferation. Chemokines are mediators of MM effects on bone and vice versa; in particular, CCL3 enhances OC formation and promotes MM cell migration and survival. Here, we characterize the effects of MLN3897, a novel specific antagonist of the chemokine receptor CCR1, on both OC formation and OC-MM cell interactions. MLN3897 demonstrates significant impairment of OC formation (by 40%) and function (by 70%), associated with decreased precursor cell multinucleation and down-regulation of c-fos signaling. OCs secrete high levels of CCL3, which triggers MM cell migration; conversely, MLN3897 abrogates its effects by inhibiting Akt signaling. Moreover, MM cell-to-OC adhesion was abrogated by MLN3897, thereby inhibiting MM cell survival and proliferation. Our results therefore show novel biologic sequelae of CCL3 and its inhibition in both osteoclastogenesis and MM cell growth, providing the preclinical rationale for clinical trials of MLN3897 to treat OBD in MM.
Figures
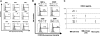

References
-
- Body JJ. Effectiveness and cost of bisphosphonate therapy in tumor bone disease. Cancer. 2003;97:859–865. - PubMed
-
- Abe M, Hiura K, Wilde J, et al. Osteoclasts enhance myeloma cell growth and survival via cell-cell contact: a vicious cycle between bone destruction and myeloma expansion. Blood. 2004;104:2484–2491. - PubMed
-
- Charo IF, Ransohoff RM. The many roles of chemokines and chemokine receptors in inflammation. N Engl J Med. 2006;354:610–621. - PubMed
-
- Tian Y, New DC, Yung LY, et al. Differential chemokine activation of CC chemokine receptor 1-regulated pathways: ligand selective activation of Galpha 14-coupled pathways. Eur J Immunol. 2004;34:785–795. - PubMed
-
- Godessart N. Chemokine receptors: attractive targets for drug discovery. Ann N Y Acad Sci. 2005;1051:647–657. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

