Direct spectroscopic evidence for a high-spin Fe(IV) intermediate in tyrosine hydroxylase
- PMID: 17715926
- PMCID: PMC2860260
- DOI: 10.1021/ja074446s
Direct spectroscopic evidence for a high-spin Fe(IV) intermediate in tyrosine hydroxylase
Figures
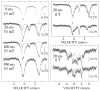

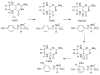

References
-
-
Abbreviations used: TyrH, tyrosine hydroxylase; Tyr, tyrosine; DOPA, dihydroxyphenylalanine; 6-MePH4, 6-methyl tetrahydropterin; 4a-HOPH3, 4a-hydroxypterin.
-
-
- Fitzpatrick PF. Ann Rev Biochem. 1999;68:355–381. - PubMed
-
- Fitzpatrick PF. In: Advances in Enzymology and Related Areas of Molecular Biology. Purich DL, editor. Vol. 74. John Wiley & Sons; New York: 2000. pp. 235–294. - PubMed
-
- Que L., Jr Nat Struct Biol. 2000;7:182–184. - PubMed
-
- Goodwill KE, Sabatier C, Marks C, Raag R, Fitzpatrick PF, Stevens RC. Nat Struct Biol. 1997;4:578–585. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

