Microtubule regulation of corneal fibroblast morphology and mechanical activity in 3-D culture
- PMID: 17716657
- PMCID: PMC2081970
- DOI: 10.1016/j.exer.2007.07.008
Microtubule regulation of corneal fibroblast morphology and mechanical activity in 3-D culture
Abstract
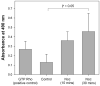
The purpose of this study was to investigate the role of microtubules in regulating corneal fibroblast structure and mechanical behavior using static (3-D) and dynamic (4-D) imaging of both cells and their surrounding matrix. Human corneal fibroblasts transfected to express GFP-zyxin (to label focal adhesions) or GFP-tubulin (to label microtubules) were plated at low density inside 100 microm thick type I collagen matrices. After 24h, the effects of nocodazole (to depolymerize microtubules), cytochalasin D (to disrupt f-actin), and/or Y-27632 (to block Rho-kinase) were evaluated using 3-D and 4-D imaging of both cells and ECM. After 24h of incubation, cells had well organized microtubules and prominent focal adhesions, and significant cell-induced matrix compaction was observed. Addition of nocodazole induced rapid microtubule disruption which resulted in Rho activation and additional cellular contraction. The matrix was pulled inward by retracting pseudopodial processes, and focal adhesions appeared to mediate this process. Following 24h exposure to nocodazole, there was an even greater increase in both the number of stress fibers and the amount of matrix compaction and alignment at the ends of cells. When Rho-kinase was inhibited, disruption of microtubules resulted in retraction of dendritic cell processes, and rapid formation and extension of lamellipodial processes at random locations along the cell body, eventually leading to a convoluted, disorganized cell shape. These data suggest that microtubules modulate both cellular contractility and local collagen matrix reorganization via regulation of Rho/Rho-kinase activity. In addition, microtubules appear to play a central role in dynamic regulation of cell spreading mechanics, morphology and polarity in 3-D culture.
Figures





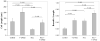
Similar articles
-
Corneal fibroblasts respond rapidly to changes in local mechanical stress.Invest Ophthalmol Vis Sci. 2004 Oct;45(10):3466-74. doi: 10.1167/iovs.04-0361. Invest Ophthalmol Vis Sci. 2004. PMID: 15452051
-
Quantitative assessment of local collagen matrix remodeling in 3-D culture: the role of Rho kinase.Exp Cell Res. 2006 Nov 1;312(18):3683-92. doi: 10.1016/j.yexcr.2006.08.009. Epub 2006 Aug 16. Exp Cell Res. 2006. PMID: 16978606 Free PMC article.
-
The Role of Thrombin and Cell Contractility in Regulating Clustering and Collective Migration of Corneal Fibroblasts in Different ECM Environments.Invest Ophthalmol Vis Sci. 2015 Mar 3;56(3):2079-90. doi: 10.1167/iovs.15-16388. Invest Ophthalmol Vis Sci. 2015. PMID: 25736789 Free PMC article.
-
Microtubule involvement in regulating cell contractility and adhesion-dependent signalling: a possible mechanism for polarization of cell motility.Biochem Soc Symp. 1999;65:147-72. Biochem Soc Symp. 1999. PMID: 10320938 Review.
-
Fibroblast mechanics in 3D collagen matrices.Adv Drug Deliv Rev. 2007 Nov 10;59(13):1299-305. doi: 10.1016/j.addr.2007.08.006. Epub 2007 Aug 14. Adv Drug Deliv Rev. 2007. PMID: 17825456 Free PMC article. Review.
Cited by
-
Impact of the clinical use of ROCK inhibitor on the pathogenesis and treatment of glaucoma.Jpn J Ophthalmol. 2018 Mar;62(2):109-126. doi: 10.1007/s10384-018-0566-9. Epub 2018 Feb 14. Jpn J Ophthalmol. 2018. PMID: 29445943 Review.
-
Techniques for assessing 3-D cell-matrix mechanical interactions in vitro and in vivo.Exp Cell Res. 2013 Oct 1;319(16):2470-80. doi: 10.1016/j.yexcr.2013.06.018. Epub 2013 Jun 29. Exp Cell Res. 2013. PMID: 23819988 Free PMC article. Review.
-
Rho-associated protein kinase inhibitor induced morphological changes in type VI collagen in the human trabecular meshwork.Br J Ophthalmol. 2020 Mar;104(3):392-397. doi: 10.1136/bjophthalmol-2018-312991. Epub 2019 Jun 14. Br J Ophthalmol. 2020. PMID: 31201167 Free PMC article.
-
Segmentation and morphometric analysis of cells from fluorescence microscopy images of cytoskeletons.Comput Math Methods Med. 2013;2013:381356. doi: 10.1155/2013/381356. Epub 2013 May 12. Comput Math Methods Med. 2013. PMID: 23762186 Free PMC article.
-
Squishy matters - Corneal mechanobiology in health and disease.Prog Retin Eye Res. 2024 Mar;99:101234. doi: 10.1016/j.preteyeres.2023.101234. Epub 2024 Jan 2. Prog Retin Eye Res. 2024. PMID: 38176611 Free PMC article. Review.
References
-
- Abbott A. Biology's new dimension. Nature. 2003;424:870–872. - PubMed
-
- Amano M, Chihara K, Nakamura N, Fukata Y, Yano T, Shibata M, Ikebe M, Kaibuchi K. Myosin II activation promotes neurite retraction during the action of Rho and Rho-kinase. Genes Cells. 1998;3:177–188. - PubMed
-
- Amano M, Ito M, Kimura K, Fukata Y, Chihara K, Nakano T, Matsuura Y, Kaibuchi K. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase) J Biol Chem. 1996;271:20246–20249. - PubMed
-
- Anderson S, DiCesare L, Tan I, Leung T, SundarRaj N. Rho-mediated assembly of stress fibers is differentially regulated in corneal fibroblats and myofibroblasts. Exp Cell Res. 2004;298:574–583. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

