Effects of anabolic androgenic steroids on the development and expression of running wheel activity and circadian rhythms in male rats
- PMID: 17716697
- PMCID: PMC4476530
- DOI: 10.1016/j.physbeh.2007.07.010
Effects of anabolic androgenic steroids on the development and expression of running wheel activity and circadian rhythms in male rats
Abstract
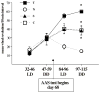
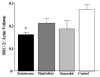
In humans, anabolic androgenic steroid (AAS) use has been associated with hyperactivity and disruption of circadian rhythmicity. We used an animal model to determine the impact of AAS on the development and expression of circadian function. Beginning on day 68 gonadally intact male rats received testosterone, nandrolone, or stanozolol via constant release pellets for 60 days; gonadally intact controls received vehicle pellets. Wheel running was recorded in a 12:12 LD cycle and constant dim red light (RR) before and after AAS implants. Post-AAS implant, circadian activity phase, period and mean level of wheel running wheel activity were compared to baseline measures. Post-AAS phase response to a light pulse at circadian time 15 h was also tested. To determine if AAS differentially affects steroid receptor coactivator (SRC) expression we measured SRC-1 and SRC-2 protein in brain. Running wheel activity was significantly elevated by testosterone, significantly depressed by nandrolone, and unaffected by stanozolol. None of the AAS altered measures of circadian rhythmicity or phase response. While SRC-1 was unaffected by AAS exposure, SRC-2 was decreased by testosterone in the hypothalamus. Activity levels, phase of peak activity and circadian period all changed over the course of development from puberty to adulthood. Development of activity was clearly modified by AAS exposure as testosterone significantly elevated activity levels and nandrolone significantly suppressed activity relative to controls. Thus, AAS exposure differentially affects both the magnitude and direction of developmental changes in activity levels depending in part on the chemical composition of the AAS.
Figures


Similar articles
-
Stacking anabolic androgenic steroids (AAS) during puberty in rats: a neuroendocrine and behavioral assessment.Pharmacol Biochem Behav. 2006 Mar;83(3):410-9. doi: 10.1016/j.pbb.2006.03.001. Epub 2006 Apr 17. Pharmacol Biochem Behav. 2006. PMID: 16603236
-
Anabolic-androgenic steroid treatment induces behavioral disinhibition and downregulation of serotonin receptor messenger RNA in the prefrontal cortex and amygdala of male mice.Genes Brain Behav. 2009 Mar;8(2):161-73. doi: 10.1111/j.1601-183X.2008.00458.x. Genes Brain Behav. 2009. PMID: 19055689
-
Effects of withdrawal from anabolic androgenic steroids on aggression in adult male rats.Physiol Behav. 2002 Apr 1;75(4):541-9. doi: 10.1016/s0031-9384(02)00657-1. Physiol Behav. 2002. PMID: 12062318
-
Impact of anabolic androgenic steroids on adolescent males.Physiol Behav. 2010 Jun 1;100(3):199-204. doi: 10.1016/j.physbeh.2010.01.007. Epub 2010 Jan 22. Physiol Behav. 2010. PMID: 20096713 Review.
-
Anabolic androgenic steroids and aggression: studies using animal models.Ann N Y Acad Sci. 2004 Dec;1036:399-415. doi: 10.1196/annals.1330.024. Ann N Y Acad Sci. 2004. PMID: 15817752 Review.
Cited by
-
Steroid receptor coactivator-2 expression in brain and physical associations with steroid receptors.Neuroscience. 2010 Sep 1;169(3):1017-28. doi: 10.1016/j.neuroscience.2010.05.053. Epub 2010 Jun 2. Neuroscience. 2010. PMID: 20678994 Free PMC article.
-
Nuclear receptor coactivators are coexpressed with steroid receptors and regulated by estradiol in mouse brain.Neuroendocrinology. 2011;94(1):49-57. doi: 10.1159/000323780. Epub 2011 Feb 9. Neuroendocrinology. 2011. PMID: 21311177 Free PMC article.
-
Modulation of steroid action in the central and peripheral nervous systems by nuclear receptor coactivators.Psychoneuroendocrinology. 2009 Dec;34 Suppl 1:S9-19. doi: 10.1016/j.psyneuen.2009.05.022. Psychoneuroendocrinology. 2009. PMID: 19541426 Free PMC article. Review.
-
Nuclear Thimet oligopeptidase is coexpressed with oestrogen receptor alpha in hypothalamic cells and regulated by oestradiol in female mice.J Neuroendocrinol. 2010 Aug;22(8):936-43. doi: 10.1111/j.1365-2826.2010.02009.x. Epub 2010 Apr 23. J Neuroendocrinol. 2010. PMID: 20456597 Free PMC article.
-
The neuroendocrine control of the circadian system: adolescent chronotype.Front Neuroendocrinol. 2012 Aug;33(3):211-29. doi: 10.1016/j.yfrne.2012.04.003. Epub 2012 May 23. Front Neuroendocrinol. 2012. PMID: 22634481 Free PMC article. Review.
References
-
- DuRant RH, Escobedo LG, Heath GW. Anabolic steroid use, strength training, and multiple drug use among adolescents in the United States. Pediatrics. 1995;96:23–28. - PubMed
-
- Haupt H, Rovere G. Anabolic steroids: a review of the literature. Am J Sports Med. 1984;12:469–489. - PubMed
-
- Lovstakken K, Peterson L, Homer AL. Risk factors for anabolic steroid use in college students and the role of expectancy. Addict Behav. 1999;24:425–430. - PubMed
-
- Stilger VG, Yesalis CE. Anabolic–androgenic steroid use among high school football players. J Community Health. 1999;24:131–145. - PubMed
-
- Uzych L. Anabolic-androgenic steroids and psychiatric-related effects: a review. Can J Psychiatry. 1992:37. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

