Specific binding of a Pop6/Pop7 heterodimer to the P3 stem of the yeast RNase MRP and RNase P RNAs
- PMID: 17717080
- PMCID: PMC1986809
- DOI: 10.1261/rna.654407
Specific binding of a Pop6/Pop7 heterodimer to the P3 stem of the yeast RNase MRP and RNase P RNAs
Abstract
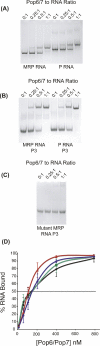
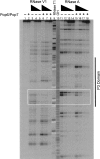
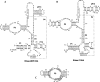
Pop6 and Pop7 are protein subunits of Saccharomyces cerevisiae RNase MRP and RNase P. Here we show that bacterially expressed Pop6 and Pop7 form a soluble heterodimer that binds the RNA components of both RNase MRP and RNase P. Footprint analysis of the interaction between the Pop6/7 heterodimer and the RNase MRP RNA, combined with gel mobility assays, demonstrates that the Pop6/7 complex binds to a conserved region of the P3 domain. Binding of these proteins to the MRP RNA leads to local rearrangement in the structure of the P3 loop and suggests that direct interaction of the Pop6/7 complex with the P3 domain of the RNA components of RNases MRP and P may mediate binding of other protein components. These results suggest a role for a key element in the RNase MRP and RNase P RNAs in protein binding, and demonstrate the feasibility of directly studying RNA-protein interactions in the eukaryotic RNases MRP and P complexes.
Figures

References
-
- Altman, S., Kirsebom, L. Ribonuclease P. In: Gesteland R.F., et al., editors. The RNA world. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1999. pp. 351–380.
-
- Forster, A.C., Altman, S. Similar cage-shaped structures for the RNA components of all Ribonuclease P and Ribonuclease MRP enzymes. Cell. 1990;62:407–409. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
