Tissue-type plasminogen activator and the low-density lipoprotein receptor-related protein mediate cerebral ischemia-induced nuclear factor-kappaB pathway activation
- PMID: 17717150
- PMCID: PMC1988877
- DOI: 10.2353/ajpath.2007.070472
Tissue-type plasminogen activator and the low-density lipoprotein receptor-related protein mediate cerebral ischemia-induced nuclear factor-kappaB pathway activation
Abstract
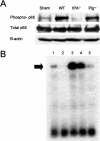
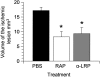
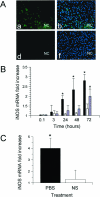
Tissue-type plasminogen activator (tPA) is a serine proteinase found in the intravascular space and the central nervous system. The low-density lipoprotein receptor-related protein (LRP) is a member of the low-density lipoprotein receptor gene family found in neurons and astrocytes. Cerebral ischemia induces activation of the nuclear factor (NF)-kappaB pathway. The present study investigated the role that the interaction between tPA and LRP plays on middle cerebral artery occlusion (MCAO)-induced NF-kappaB-mediated inflammatory response. We found that MCAO increased LRP expression primarily in astrocytes and that this effect was significantly decreased in the absence of tPA. The onset of the ischemic insult induced activation of the NF-kappaB pathway in wild-type and plasminogen (Plg(-/-))-deficient mice, and this effect was attenuated after inhibition of LRP or genetic deficiency of tPA. Moreover, administration of tPA to tPA(-/-) mice resulted in activation of the NF-kappaB pathway comparable with that observed in wild-type and Plg(-/-) mice. We also report that inhibition of either tPA activity or LRP or genetic deficiency of tPA resulted in a significant decrease in MCAO-induced nitric oxide production and inducible nitric-oxide synthase expression. In conclusion, our results demonstrate that after MCAO the interaction between tPA and LRP results in NF-kappaB activation in astrocytes and induction of inducible nitric-oxide synthase expression in the ischemic tissue, suggesting a cytokine-like plasminogen-independent role for tPA during cerebral ischemia.
Figures


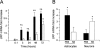


Similar articles
-
The low-density lipoprotein receptor-related protein 1 mediates tissue-type plasminogen activator-induced microglial activation in the ischemic brain.Am J Pathol. 2009 Feb;174(2):586-94. doi: 10.2353/ajpath.2009.080661. Epub 2009 Jan 15. Am J Pathol. 2009. PMID: 19147818 Free PMC article.
-
Microglial low-density lipoprotein receptor-related protein 1 mediates the effect of tissue-type plasminogen activator on matrix metalloproteinase-9 activity in the ischemic brain.J Cereb Blood Flow Metab. 2009 Dec;29(12):1946-54. doi: 10.1038/jcbfm.2009.174. Epub 2009 Aug 12. J Cereb Blood Flow Metab. 2009. PMID: 19672275
-
Tissue-type plasminogen activator-mediated shedding of astrocytic low-density lipoprotein receptor-related protein increases the permeability of the neurovascular unit.Blood. 2007 Apr 15;109(8):3270-8. doi: 10.1182/blood-2006-08-043125. Epub 2006 Dec 14. Blood. 2007. PMID: 17170123 Free PMC article.
-
Role of tissue-type plasminogen activator in ischemic stroke.J Pharmacol Sci. 2010;113(3):203-7. doi: 10.1254/jphs.10r01cp. Epub 2010 Jun 29. J Pharmacol Sci. 2010. PMID: 20595786 Review.
-
Reprint of: Fibrinolytic and Non-fibrinolytic Roles of Tissue-type Plasminogen Activator in the Ischemic Brain.Neuroscience. 2024 Jul 9;550:21-29. doi: 10.1016/j.neuroscience.2024.05.040. Epub 2024 Jul 2. Neuroscience. 2024. PMID: 38964373 Review.
Cited by
-
Regulated intramembrane proteolysis of the low-density lipoprotein receptor-related protein mediates ischemic cell death.Am J Pathol. 2008 May;172(5):1355-62. doi: 10.2353/ajpath.2008.070975. Epub 2008 Apr 10. Am J Pathol. 2008. PMID: 18403601 Free PMC article.
-
The low-density lipoprotein receptor-related protein 1 mediates tissue-type plasminogen activator-induced microglial activation in the ischemic brain.Am J Pathol. 2009 Feb;174(2):586-94. doi: 10.2353/ajpath.2009.080661. Epub 2009 Jan 15. Am J Pathol. 2009. PMID: 19147818 Free PMC article.
-
Therapeutic Strategies to Attenuate Hemorrhagic Transformation After Tissue Plasminogen Activator Treatment for Acute Ischemic Stroke.J Atheroscler Thromb. 2017 Mar 1;24(3):240-253. doi: 10.5551/jat.RV16006. Epub 2016 Dec 13. J Atheroscler Thromb. 2017. PMID: 27980241 Free PMC article. Review.
-
Tissue-type plasminogen activator protects neurons from excitotoxin-induced cell death via activation of the ERK1/2-CREB-ATF3 signaling pathway.Mol Cell Neurosci. 2013 Jan;52:9-19. doi: 10.1016/j.mcn.2012.10.001. Epub 2012 Oct 9. Mol Cell Neurosci. 2013. PMID: 23063501 Free PMC article.
-
tPA activates LDL receptor-related protein 1-mediated mitogenic signaling involving the p90RSK and GSK3beta pathway.Am J Pathol. 2010 Oct;177(4):1687-96. doi: 10.2353/ajpath.2010.100213. Epub 2010 Aug 19. Am J Pathol. 2010. PMID: 20724593 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

