Effects of 1-year treatment with cyclophosphamide on outcomes at 2 years in scleroderma lung disease
- PMID: 17717203
- PMCID: PMC2078679
- DOI: 10.1164/rccm.200702-326OC
Effects of 1-year treatment with cyclophosphamide on outcomes at 2 years in scleroderma lung disease
Abstract
Rationale: The Scleroderma Lung Study enrolled 158 patients with scleroderma-related interstitial lung disease in a placebo-controlled trial of oral cyclophosphamide (CYC). Although treatment-related benefits in pulmonary function, skin scores, and patient-centered outcomes were demonstrated after 1 year of therapy, the duration of benefit beyond 1 year was unclear.
Objectives: A second year of follow-up was performed to determine if these effects persisted after stopping treatment.
Methods: A detailed analysis of data obtained over the two years of the study was performed.
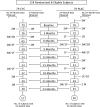
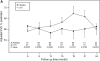
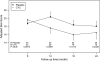
Measurements and main results: Using a longitudinal joint model, we analyzed FVC, total lung capacity, transitional dyspnea index, Rodnan skin scores, and the Health Assessment Questionnaire-Disability Index during the second year, after adjusting for baseline values, baseline fibrosis score, and nonignorable missing data. Evaluable subjects (72 CYC; 73 placebo) included 93 who completed all visits plus 52 who completed at least 6 months of therapy and returned at 24 month or had their 24-month data imputed. The beneficial effects of CYC on pulmonary function and health status continued to increase through 18 months, after which they dissipated, whereas skin improvements dissipated after 12 months. In contrast, the positive effect on dyspnea persisted through 24 months. Adverse events were uncommon.
Conclusions: One year of CYC improved lung function, skin scores, dyspnea, and health status/disability, effects which either persisted or increased further for several months after stopping therapy. However, except for a sustained impact on dyspnea, all of these effects waned and were no longer apparent at 24 months. Treatment strategies aimed at extending the positive therapeutic effects observed with CYC should be considered. Clinical trial registered with www.clinicaltrials.gov (NCT 000004563).
Trial registration: ClinicalTrials.gov NCT00004563.
Figures


Comment in
-
Daily cyclophosphamide for scleroderma: are patients with the most to gain underrepresented in this trial?Am J Respir Crit Care Med. 2007 Nov 15;176(10):952-3. doi: 10.1164/rccm.200708-1185ED. Am J Respir Crit Care Med. 2007. PMID: 17984310 No abstract available.
References
-
- Steen VD, Conte C, Owens GR, Medsger TA Jr. Severe restrictive lung disease in systemic sclerosis. Arthritis Rheum 1994;37:1283–1289. - PubMed
-
- Mayes MD, Reveille JD. Epidemiology, demographics, and genetics. In: PJ Clements, DE Furst, editors. Systemic sclerosis, 2nd ed. New York: Lippincott, Williams, & Wilkins; 2004. pp. 1–15.
-
- Silver RM, Warrick JH, Kinsella MB, Staudt LS, Baumann MH, Strange C. Cyclophosphamide and low-dose prednisone therapy in patients with systemic sclerosis (scleroderma) with interstitial lung disease. J Rheumatol 1993;20:834–844. - PubMed
-
- Akesson A, Scheja A, Lundin A, Wollheim F. Improved pulmonary function in systemic sclerosis after treatment with cyclophosphamide. Arthritis Rheum 1994;37:729–735. - PubMed
-
- Steen VD, Lanz JK Jr, Conte C, Owens GR, Medsger TA Jr. Therapy for severe interstitial lung disease in systemic sclerosis: a retrospective study. Arthritis Rheum 1994;37:1290–1296. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

