The specificity of SNARE pairing in biological membranes is mediated by both proof-reading and spatial segregation
- PMID: 17717530
- PMCID: PMC1994121
- DOI: 10.1038/sj.emboj.7601820
The specificity of SNARE pairing in biological membranes is mediated by both proof-reading and spatial segregation
Abstract
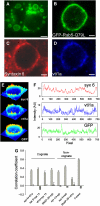
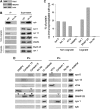
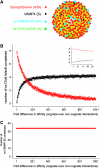
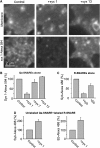
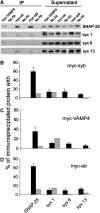
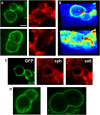
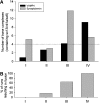
Soluble N-ethylmaleimide-sensitive factor attachment receptor (SNARE) proteins mediate organelle fusion in the secretory pathway. Different fusion steps are catalyzed by specific sets of SNARE proteins. Here we have used the SNAREs mediating the fusion of early endosomes and exocytosis, respectively, to investigate how pairing specificity is achieved. Although both sets of SNAREs promiscuously assemble in vitro, there is no functional crosstalk. We now show that they not only colocalize to overlapping microdomains in the membrane of early endosomes of neuroendocrine cells, but also form cis-complexes promiscuously, with the proportion of the different complexes being primarily dependent on mass action. Addition of soluble SNARE molecules onto native membranes revealed preference for cognate SNAREs. Furthermore, we found that SNAREs are laterally segregated at endosome contact sites, with the exocytotic synaptobrevin being depleted. We conclude that specificity in endosome fusion is mediated by the following two synergistically operating mechanisms: (i) preference for the cognate SNARE in 'trans' interactions and (ii) lateral segregation of SNAREs, leading to relative enrichment of the cognate ones at the prospective fusion sites.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

