Molecular basis of the activity of the phytopathogen pectin methylesterase
- PMID: 17717531
- PMCID: PMC2000356
- DOI: 10.1038/sj.emboj.7601816
Molecular basis of the activity of the phytopathogen pectin methylesterase
Abstract
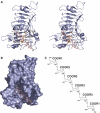
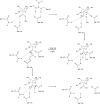
We provide a mechanism for the activity of pectin methylesterase (PME), the enzyme that catalyses the essential first step in bacterial invasion of plant tissues. The complexes formed in the crystal using specifically methylated pectins, together with kinetic measurements of directed mutants, provide clear insights at atomic resolution into the specificity and the processive action of the Erwinia chrysanthemi enzyme. Product complexes provide additional snapshots along the reaction coordinate. We previously revealed that PME is a novel aspartic-esterase possessing parallel beta-helix architecture and now show that the two conserved aspartates are the nucleophile and general acid-base in the mechanism, respectively. Other conserved residues at the catalytic centre are shown to be essential for substrate binding or transition state stabilisation. The preferential binding of methylated sugar residues upstream of the catalytic site, and demethylated residues downstream, drives the enzyme along the pectin molecule and accounts for the sequential pattern of demethylation produced by both bacterial and plant PMEs.
Figures

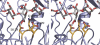
References
-
- Arabidopsis Initiative (2000) Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature 408: 796–815 - PubMed
-
- CCP4 Initiative (1994) The CCP4 suite: programs for protein crystallography. Acta Crystallogr D 50: 760–763 - PubMed
-
- Christensen TMIE, Brunstedt J, Buchholt HC, Mikkelsen JD (2001) Pectins and Pectinases. The Netherlands: Rotterdam
-
- Clausen M, Madsen R (2004) Synthesis of oligogalacturonates conjugated to BSA. Carbohydr Res 339: 2159–2169 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

