Discovery of common human genetic variants of GTP cyclohydrolase 1 (GCH1) governing nitric oxide, autonomic activity, and cardiovascular risk
- PMID: 17717598
- PMCID: PMC1950457
- DOI: 10.1172/JCI31093
Discovery of common human genetic variants of GTP cyclohydrolase 1 (GCH1) governing nitric oxide, autonomic activity, and cardiovascular risk
Abstract
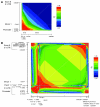
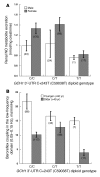
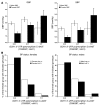
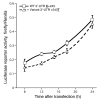
GTP cyclohydrolase 1 (GCH1) is rate limiting in the provision of the cofactor tetrahydrobiopterin for biosynthesis of catecholamines and NO. We asked whether common genetic variation at GCH1 alters transmitter synthesis and predisposes to disease. Here we undertook a systematic search for polymorphisms in GCH1, then tested variants' contributions to NO and catecholamine release as well as autonomic function in twin pairs. Renal NO and neopterin excretions were significantly heritable, as were baroreceptor coupling (heart rate response to BP fluctuation) and pulse interval (1/heart rate). Common GCH1 variant C+243T in the 3'-untranslated region (3'-UTRs) predicted NO excretion, as well as autonomic traits: baroreceptor coupling, maximum pulse interval, and pulse interval variability, though not catecholamine secretion. In individuals with the most extreme BP values in the population, C+243T affected both diastolic and systolic BP, principally in females. In functional studies, C+243T decreased reporter expression in transfected 3'-UTRs plasmids. We conclude that human NO secretion traits are heritable, displaying joint genetic determination with autonomic activity by functional polymorphism at GCH1. Our results document novel pathophysiological links between a key biosynthetic locus and NO metabolism and suggest new strategies for approaching the mechanism, diagnosis, and treatment of risk predictors for cardiovascular diseases such as hypertension.
Figures

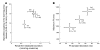

References
-
- Serova L., Nankova B., Rivkin M., Kvetnansky R., Sabban E.L. Glucocorticoids elevate GTP cyclohydrolase I mRNA levels in vivo and in PC12 cells. Brain Res. Mol. Brain Res. 1997;48:251–258. - PubMed
-
- Furukawa Y., et al. Dystonia with motor delay in compound heterozygotes for GTP-cyclohydrolase I gene mutations. Ann. Neurol. 1998;44:10–16. - PubMed
-
- Hyland K., Gunasekara R.S., Munk-Martin T.L., Arnold L.A., Engle T. The hph-1 mouse: a model for dominantly inherited GTP-cyclohydrolase deficiency. Ann. Neurol. 2003;54(Suppl. 6):S46–S48. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

