Alcohol metabolism's damaging effects on the cell: a focus on reactive oxygen generation by the enzyme cytochrome P450 2E1
- PMID: 17718406
- PMCID: PMC6527031
Alcohol metabolism's damaging effects on the cell: a focus on reactive oxygen generation by the enzyme cytochrome P450 2E1
Abstract
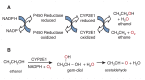
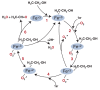
Alcohol metabolism's various processes create harmful compounds that contribute to cell and tissue damage. In particular, the enzyme cytochrome P450 2E1 (CYP2E1) plays a role in creating a harmful condition known as oxidative stress. This condition is related to oxygen's ability to accept electrons and the subsequent highly reactive and harmful byproducts created by these chemical reactions. CYP2E1's use of oxygen in alcohol metabolism generates reactive oxygen species, ultimately leading to oxidative stress and tissue damage.
Conflict of interest statement
The author declares that he has no competing financial interests.
Figures

Similar articles
-
Ethanol metabolism by alcohol dehydrogenase or cytochrome P450 2E1 differentially impairs hepatic protein trafficking and growth hormone signaling.Am J Physiol Gastrointest Liver Physiol. 2017 Dec 1;313(6):G558-G569. doi: 10.1152/ajpgi.00027.2017. Epub 2017 Sep 1. Am J Physiol Gastrointest Liver Physiol. 2017. PMID: 28864499 Free PMC article.
-
Hepatitis C virus core protein, cytochrome P450 2E1, and alcohol produce combined mitochondrial injury and cytotoxicity in hepatoma cells.Gastroenterology. 2005 Jan;128(1):96-107. doi: 10.1053/j.gastro.2004.10.045. Gastroenterology. 2005. PMID: 15633127
-
Absence of cytochrome P450 2A5 enhances alcohol-induced liver injury in mice.Dig Liver Dis. 2015 Jun;47(6):470-7. doi: 10.1016/j.dld.2015.02.012. Epub 2015 Mar 6. Dig Liver Dis. 2015. PMID: 25804444 Free PMC article.
-
Microsomal Ethanol-Oxidizing System: Success Over 50 Years and an Encouraging Future.Alcohol Clin Exp Res. 2019 Mar;43(3):386-400. doi: 10.1111/acer.13961. Epub 2019 Feb 11. Alcohol Clin Exp Res. 2019. PMID: 30667528 Review.
-
The role of reactive oxygen species (ROS) and cytochrome P-450 2E1 in the generation of carcinogenic etheno-DNA adducts.Redox Biol. 2014;3:56-62. doi: 10.1016/j.redox.2014.08.009. Epub 2014 Sep 6. Redox Biol. 2014. PMID: 25462066 Free PMC article. Review.
Cited by
-
Exploring Nutritional Status and Metabolic Imbalances in Children with FASD: A Cross-Sectional Study.Nutrients. 2024 Oct 7;16(19):3401. doi: 10.3390/nu16193401. Nutrients. 2024. PMID: 39408368 Free PMC article.
-
Prenatal and adolescent alcohol exposure programs immunity across the lifespan: CNS-mediated regulation.Pharmacol Biochem Behav. 2022 May;216:173390. doi: 10.1016/j.pbb.2022.173390. Epub 2022 Apr 18. Pharmacol Biochem Behav. 2022. PMID: 35447157 Free PMC article. Review.
-
Oxidant Stress and Acetaminophen Hepatotoxicity: Mechanism-Based Drug Development.Antioxid Redox Signal. 2021 Sep 20;35(9):718-733. doi: 10.1089/ars.2021.0102. Epub 2021 Jul 7. Antioxid Redox Signal. 2021. PMID: 34232786 Free PMC article.
-
Role of IRAK-M in alcohol induced liver injury.PLoS One. 2013;8(2):e57085. doi: 10.1371/journal.pone.0057085. Epub 2013 Feb 21. PLoS One. 2013. PMID: 23437317 Free PMC article.
-
Heme oxygenase-1 prevents non-alcoholic steatohepatitis through suppressing hepatocyte apoptosis in mice.Lipids Health Dis. 2010 Oct 28;9:124. doi: 10.1186/1476-511X-9-124. Lipids Health Dis. 2010. PMID: 20979658 Free PMC article.
References
-
- Nelson DR. Cytochrome P450 nomenclature. Methods in Molecular Biology. 1998;107:15–24. - PubMed
-
- Nelson DR, Zeldin DC, Hoffman SM, et al. Comparison of cytochrome P450 (CYP) genes from the mouse and human genomes, including nomenclature recommendations for genes, pseudogenes and alternative-splice variants. Pharmacogenetics. 2004;14:1–18. - PubMed
-
- Dey A, Cederbaum AI. Alcohol and oxidative liver injury. Hepatology. 2006;43(Suppl 1):S63–S74. - PubMed
-
- Gonzalez FJ. Role of cytochromes P450 in chemical toxicity and oxidative stress: Studies with CYP2E1. Mutation Research. 2005;569:101–110. - PubMed
-
- Guengerich FP. Cytochromes P450, drugs, and diseases. Molecular Interventions. 2003;3:194–204. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
