Lipid rafts and B cell signaling
- PMID: 17719248
- PMCID: PMC2169358
- DOI: 10.1016/j.semcdb.2007.07.009
Lipid rafts and B cell signaling
Abstract
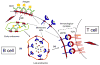
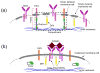
B cells comprise an essential component of the humoral immune system. They are equipped with the unique ability to synthesize and secrete pathogen-neutralizing antibodies, and share with professional antigen presenting cells the ability to internalize foreign antigens, and process them for presentation to helper T cells. Recent evidence indicates that specialized cholesterol- and glycosphingolipid-rich microdomains in the plasma membrane commonly referred to as lipid rafts, serve to compartmentalize key signaling molecules during the different stages of B cell activation including B cell antigen receptor (BCR)-initiated signal transduction, endocytosis of BCR-antigen complexes, loading of antigenic peptides onto MHC class II molecules, MHC-II associated antigen presentation to helper T cells, and receipt of helper signals via the CD40 receptor. Here we review the recent literature arguing for a role of lipid rafts in the spatial organization of B cell function.
Figures

Similar articles
-
Floating the raft hypothesis: the roles of lipid rafts in B cell antigen receptor function.Semin Immunol. 2001 Apr;13(2):107-14. doi: 10.1006/smim.2000.0302. Semin Immunol. 2001. PMID: 11308294 Review.
-
Tetraspanin microdomains in immune cell signalling and malignant disease.Tissue Antigens. 2004 Nov;64(5):533-42. doi: 10.1111/j.1399-0039.2004.00321.x. Tissue Antigens. 2004. PMID: 15496196 Review.
-
Floating the raft hypothesis for immune receptors: access to rafts controls receptor signaling and trafficking.Traffic. 2001 Mar;2(3):160-6. doi: 10.1034/j.1600-0854.2001.020302.x. Traffic. 2001. PMID: 11260521 Review.
-
Lipid rafts in T cell signalling and disease.Semin Cell Dev Biol. 2007 Oct;18(5):608-15. doi: 10.1016/j.semcdb.2007.08.002. Epub 2007 Aug 19. Semin Cell Dev Biol. 2007. PMID: 17890113 Free PMC article. Review.
-
A role for lipid rafts in B cell antigen receptor signaling and antigen targeting.J Exp Med. 1999 Dec 6;190(11):1549-60. doi: 10.1084/jem.190.11.1549. J Exp Med. 1999. PMID: 10587346 Free PMC article.
Cited by
-
Cholesterol metabolism in innate and adaptive response.F1000Res. 2018 Oct 16;7:F1000 Faculty Rev-1647. doi: 10.12688/f1000research.15500.1. eCollection 2018. F1000Res. 2018. PMID: 30364153 Free PMC article. Review.
-
B cell activation by outer membrane vesicles--a novel virulence mechanism.PLoS Pathog. 2010 Jan 15;6(1):e1000724. doi: 10.1371/journal.ppat.1000724. PLoS Pathog. 2010. PMID: 20090836 Free PMC article.
-
HGAL localization to cell membrane regulates B-cell receptor signaling.Blood. 2015 Jan 22;125(4):649-57. doi: 10.1182/blood-2014-04-571331. Epub 2014 Nov 7. Blood. 2015. PMID: 25381061 Free PMC article.
-
Novel drug targets for personalized precision medicine in relapsed/refractory diffuse large B-cell lymphoma: a comprehensive review.Mol Cancer. 2015 Dec 11;14:207. doi: 10.1186/s12943-015-0474-2. Mol Cancer. 2015. PMID: 26654227 Free PMC article. Review.
-
Aberrant localization of apoptosis protease activating factor-1 in lipid raft sub-domains of diffuse large B cell lymphomas.Oncotarget. 2016 Dec 20;7(51):83964-83975. doi: 10.18632/oncotarget.13336. Oncotarget. 2016. PMID: 27863378 Free PMC article.
References
-
- Monroe JG. ITAM-mediated tonic signalling through pre-BCR and BCR complexes. Nat Rev Immunol. 2006;6:283–94. - PubMed
-
- Reth M, Brummer T. Feedback regulation of lymphocyte signaling. Nat Rev Immunol. 2004;4:269–77. - PubMed
-
- Law DA, Chan VWF, Datta SK, DeFranco AL. B-cell antigen receptor motifs have redundant signalling capabilities and bind the tyrosine kinases PTK72, Lyn and Fyn. Curr Biol. 1993;3:645–57. - PubMed
-
- Pike LJ. Rafts defined: a report on the Keystone Symposium on Lipid Rafts and Cell Function. J Lipid Res. 2006;47:1597–8. - PubMed
-
- Dykstra M, Cherukuri A, Sohn HW, Tzeng SJ, Pierce SK. Location is everything: lipid rafts and immune cell signaling. Annu Rev Immunol. 2003;21:457–81. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

