Rapid identification of enzyme variants for reengineered alkaloid biosynthesis in periwinkle
- PMID: 17719488
- PMCID: PMC2700010
- DOI: 10.1016/j.chembiol.2007.07.008
Rapid identification of enzyme variants for reengineered alkaloid biosynthesis in periwinkle
Abstract
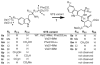
Monoterpene indole alkaloids from Catharanthus roseus (Madagascar periwinkle), such as the anticancer agents vinblastine and vincristine, have important pharmacological activities. Metabolic engineering of alkaloid biosynthesis can provide an efficient and environmentally friendly route to analogs of these synthetically challenging and pharmaceutically valuable natural products. However, the narrow substrate scope of strictosidine synthase, the enzyme at the entry point of the pathway, limits a pathway engineering approach. We demonstrate that with a different expression system and screening method it is possible to rapidly identify strictosidine synthase variants that accept tryptamine analogs not turned over by the wild-type enzyme. The variants are used in stereoselective synthesis of beta-carboline analogs and are assessed for biosynthetic competence within the terpene indole alkaloid pathway. These results present an opportunity to explore metabolic engineering of "unnatural" product production in the plant periwinkle.
Figures
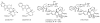
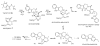
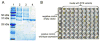
Comment in
-
Foundations for directed alkaloid biosynthesis.Chem Biol. 2007 Aug;14(8):875-6. doi: 10.1016/j.chembiol.2007.08.001. Chem Biol. 2007. PMID: 17719485
Similar articles
-
Metabolic reprogramming of periwinkle plant culture.Nat Chem Biol. 2009 Mar;5(3):151-3. doi: 10.1038/nchembio.141. Epub 2009 Jan 18. Nat Chem Biol. 2009. PMID: 19151732 Free PMC article.
-
Directed biosynthesis of alkaloid analogs in the medicinal plant Catharanthus roseus.J Am Chem Soc. 2006 Nov 8;128(44):14276-7. doi: 10.1021/ja066787w. J Am Chem Soc. 2006. PMID: 17076499
-
Aza-tryptamine substrates in monoterpene indole alkaloid biosynthesis.Chem Biol. 2009 Dec 24;16(12):1225-9. doi: 10.1016/j.chembiol.2009.11.016. Chem Biol. 2009. PMID: 20064432 Free PMC article.
-
Engineering Catharanthus roseus monoterpenoid indole alkaloid pathway in yeast.Appl Microbiol Biotechnol. 2022 Apr;106(7):2337-2347. doi: 10.1007/s00253-022-11883-5. Epub 2022 Mar 25. Appl Microbiol Biotechnol. 2022. PMID: 35333954 Review.
-
Terpenoid indole alkaloid biosynthesis in Catharanthus roseus: effects and prospects of environmental factors in metabolic engineering.Biotechnol Lett. 2021 Nov;43(11):2085-2103. doi: 10.1007/s10529-021-03179-x. Epub 2021 Sep 25. Biotechnol Lett. 2021. PMID: 34564757 Free PMC article. Review.
Cited by
-
Biosynthesis and synthetic biology of psychoactive natural products.Chem Soc Rev. 2021 Jun 21;50(12):6950-7008. doi: 10.1039/d1cs00065a. Chem Soc Rev. 2021. PMID: 33908526 Free PMC article. Review.
-
Engineered Production of Strictosidine and Analogues in Yeast.ACS Synth Biol. 2022 Apr 15;11(4):1639-1649. doi: 10.1021/acssynbio.2c00037. Epub 2022 Mar 16. ACS Synth Biol. 2022. PMID: 35294193 Free PMC article.
-
Production platforms for the molecular pharming of alkaloid diversity.Proc Natl Acad Sci U S A. 2008 Jun 10;105(23):7897-8. doi: 10.1073/pnas.0803930105. Epub 2008 Jun 9. Proc Natl Acad Sci U S A. 2008. PMID: 18541922 Free PMC article. No abstract available.
-
Expression, crystallization and preliminary X-ray analysis of McbB, a multifunctional enzyme involved in β-carboline skeleton biosynthesis.Acta Crystallogr F Struct Biol Commun. 2014 Oct;70(Pt 10):1402-5. doi: 10.1107/S2053230X14018743. Epub 2014 Sep 25. Acta Crystallogr F Struct Biol Commun. 2014. PMID: 25286949 Free PMC article.
-
Inverted Binding of Non-natural Substrates in Strictosidine Synthase Leads to a Switch of Stereochemical Outcome in Enzyme-Catalyzed Pictet-Spengler Reactions.J Am Chem Soc. 2020 Jan 15;142(2):792-800. doi: 10.1021/jacs.9b08704. Epub 2020 Jan 7. J Am Chem Soc. 2020. PMID: 31909617 Free PMC article.
References
-
- van der Heijden R, Jacobs DI, Snoeijer W, Didier H, Verpoorte R. The Catharanthus alkaloids: Pharmacognosy and biotechnology. Curr Med Chem. 2004;11:607–628. - PubMed
-
- O’Connor SE, Maresh J. Chemistry and biology of monoterpene indole alkaloid biosynthesis. Nat Prod Rep. 2006;23:532–547. - PubMed
-
- Johnson IS, Wright HF, Svoboda GH. Experimental basis for clinical evaluation of antitumor principles from Vinca rosea Linn. J Lab Clin Med. 1959;54:830–838.
-
- Svoboda GH. Alkaloids of Vinca rosea Linn. (Catharanthus roseus) 1X: Extraction and characterization of leurosidine and leurocristine. Llyodia. 1961;24:173–178.
-
- Fahy J. Modifications in the “upper” or velbenamine part of the vinca alkaloids have major implications for tubulin interacting activities. Curr Pharm Des. 2001;7:1181–1197. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

