Cooperative activation of Src family kinases by SH3 and SH2 ligands
- PMID: 17719722
- PMCID: PMC2045694
- DOI: 10.1016/j.canlet.2007.07.012
Cooperative activation of Src family kinases by SH3 and SH2 ligands
Abstract
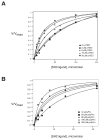
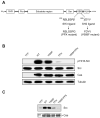
Src family nonreceptor tyrosine kinases are kept in a repressed state by intramolecular interactions involving the SH3 and SH2 domains of the enzymes. Ligands for these domains can displace the intramolecular associations and activate the kinases. Here, we carried out in vitro activation experiments with purified, down-regulated hematopoietic cell kinase (Hck), a Src family kinase. We show that SH3 and SH2 ligands act cooperatively to activate Src family kinases: the presence of one ligand lowers the concentration of the second ligand necessary for activation. To confirm the findings in intact cells, we studied Cas, a Src substrate that possesses SH2 and SH3 ligands. In contrast to wild-type Cas, mutant forms of Cas lacking the SH3 or SH2 ligands were unable to stimulate Src autophosphorylation when expressed in Cas-deficient fibroblasts. Cells expressing the Cas mutants also showed decreased amounts of activated Src at focal adhesions. The results suggest that proteins containing ligands for both SH3 and SH2 domains can produce a synergistic activation of Src family kinases.
Figures

Similar articles
-
Biochemical properties of the Cdc42-associated tyrosine kinase ACK1. Substrate specificity, authphosphorylation, and interaction with Hck.J Biol Chem. 2003 Nov 28;278(48):47713-23. doi: 10.1074/jbc.M306716200. Epub 2003 Sep 22. J Biol Chem. 2003. PMID: 14506255
-
SH3-dependent stimulation of Src-family kinase autophosphorylation without tail release from the SH2 domain in vivo.Nat Struct Biol. 2002 May;9(5):365-9. doi: 10.1038/nsb782. Nat Struct Biol. 2002. PMID: 11976726
-
Crystal structure of the Src family kinase Hck SH3-SH2 linker regulatory region supports an SH3-dominant activation mechanism.J Biol Chem. 2010 Nov 12;285(46):35455-61. doi: 10.1074/jbc.M110.145102. Epub 2010 Sep 1. J Biol Chem. 2010. PMID: 20810664 Free PMC article.
-
Src protein-tyrosine kinase structure and regulation.Biochem Biophys Res Commun. 2004 Nov 26;324(4):1155-64. doi: 10.1016/j.bbrc.2004.09.171. Biochem Biophys Res Commun. 2004. PMID: 15504335 Review.
-
Structures of Src-family tyrosine kinases.Curr Opin Struct Biol. 1997 Dec;7(6):777-85. doi: 10.1016/s0959-440x(97)80146-7. Curr Opin Struct Biol. 1997. PMID: 9434895 Review.
Cited by
-
Fyn nanoclustering requires switching to an open conformation and is enhanced by FTLD-Tau biomolecular condensates.Mol Psychiatry. 2023 Feb;28(2):946-962. doi: 10.1038/s41380-022-01825-y. Epub 2022 Oct 18. Mol Psychiatry. 2023. PMID: 36258016 Free PMC article.
-
MUC1-mediated motility in breast cancer: a review highlighting the role of the MUC1/ICAM-1/Src signaling triad.Clin Exp Metastasis. 2015 Apr;32(4):393-403. doi: 10.1007/s10585-015-9711-8. Epub 2015 Mar 11. Clin Exp Metastasis. 2015. PMID: 25759211 Review.
-
A selective NMR probe to monitor the conformational transition from inactive to active kinase.ACS Chem Biol. 2015 Jan 16;10(1):262-8. doi: 10.1021/cb5004702. Epub 2014 Sep 26. ACS Chem Biol. 2015. PMID: 25248068 Free PMC article.
-
The evolutionarily conserved arrangement of domains in SRC family kinases is important for substrate recognition.Biochemistry. 2008 Oct 14;47(41):10871-80. doi: 10.1021/bi800930e. Epub 2008 Sep 20. Biochemistry. 2008. PMID: 18803405 Free PMC article.
-
Cellular settings mediating Src Substrate switching between focal adhesion kinase tyrosine 861 and CUB-domain-containing protein 1 (CDCP1) tyrosine 734.J Biol Chem. 2011 Dec 9;286(49):42303-42315. doi: 10.1074/jbc.M111.227462. Epub 2011 Oct 12. J Biol Chem. 2011. PMID: 21994943 Free PMC article.
References
-
- Dueber JE, Yeh BJ, Bhattacharyya RP, Lim WA. Rewiring cell signaling: the logic and plasticity of eukaryotic protein circuitry. Curr Opin Struct Biol. 2004;14:690–699. - PubMed
-
- Prehoda KE, Scott JA, Mullins RD, Lim WA. Integration of multiple signals through cooperative regulation of the N-WASP-Arp2/3 complex. Science. 2000;290:801–806. - PubMed
-
- Parsons SJ, Parsons JT. Src family kinases, key regulators of signal transduction. Oncogene. 2004;23:7906–7909. - PubMed
-
- Brown MT, Cooper JA. Regulation, substrates and functions of src. Biochim Biophys Acta. 1996;1287:121–149. - PubMed
-
- Thomas SM, Brugge JS. Cellular functions regulated by Src family kinases. Annu Rev Cell Dev Biol. 1997;13:513–609. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

