Mammary epithelial cell: influence of extracellular matrix composition and organization during development and tumorigenesis
- PMID: 17719831
- PMCID: PMC2658720
- DOI: 10.1016/j.biocel.2007.06.025
Mammary epithelial cell: influence of extracellular matrix composition and organization during development and tumorigenesis
Abstract
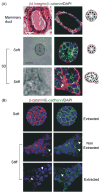
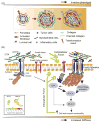
Stromal-epithelial interactions regulate mammary gland development and are critical for the maintenance of tissue homeostasis. The extracellular matrix, which is a proteinaceous component of the stroma, regulates mammary epithelial growth, survival, migration and differentiation through a repertoire of transmembrane receptors, of which integrins are the best characterized. Integrins modulate cell fate by reciprocally transducing biochemical and biophysical cues between the cell and the extracellular matrix, facilitating processes such as embryonic branching morphogenesis and lactation in the mammary gland. During breast development and cancer progression, the extracellular matrix is dynamically altered such that its composition, turnover, processing and orientation change dramatically. These modifications influence mammary epithelial cell shape, and modulate growth factor and hormonal responses to regulate processes including branching morphogenesis and alveolar differentiation. Malignant transformation of the breast is also associated with significant matrix remodeling and a progressive stiffening of the stroma that can enhance mammary epithelial cell growth, perturb breast tissue organization, and promote cell invasion and survival. In this review, we discuss the role of stromal-epithelial interactions in normal and malignant mammary epithelial cell behavior. We specifically focus on how dynamic modulation of the biochemical and biophysical properties of the extracellular matrix elicit a dialogue with the mammary epithelium through transmembrane integrin receptors to influence tissue morphogenesis, homeostasis and malignant transformation.
Figures
References
-
- Atkinson C, Warren R, Bingham SA, Day NE. Mammographic patterns as a predictive biomarker of breast cancer risk: Effect of tamoxifen. Cancer Epidemiology Biomarkers & Prevention. 1999;8:863–866. - PubMed
-
- Condeelis J, Segall JE. Intravital imaging of cell movement in tumours. Nature Reviews Cancer. 2003;3:921–930. - PubMed
-
- Cunha GR, Young P, Christov K, Guzman R, Nandi S, Talamantes F, et al. Mammary phenotypic expression induced in epidermal cells by embryonic mammary mesenchyme. Acta Anatomica (Basel) 1995;152:195–204. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

